A first-order, heterogeneous, irreversible reaction is taking place within a catalyst pore that is plated with platinum
Question:
A first-order, heterogeneous, irreversible reaction is taking place within a catalyst pore that is plated with platinum entirely along the length of the pore (Figure P15-7B). The reactant concentration at the plane of symmetry (i.e., equal distance from the pore mouth) of the pore is equal to one-tenth the concentration at the pore mouth. The concentration at the pore mouth is 0.001 mol/dm3, the pore length (2L) is 2 × 10–3 cm, and the diffusion coefficient is 0.1 cm2/s.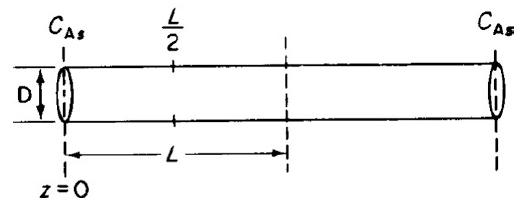
Single catalyst pore.
a. Derive an equation for the effectiveness factor.
b. What is the concentration of reactant at L/2?
c. To what length should the pore length be reduced if the effectiveness factor is to be 0.8?
d. If the catalyst support were not yet plated with platinum, how would you suggest the catalyst support be plated after the pore length, L, had been reduced by grinding?
Step by Step Answer:






