A small reaction bomb fitted with a sensitive pressure-measuring device is flushed out and then filled with
Question:
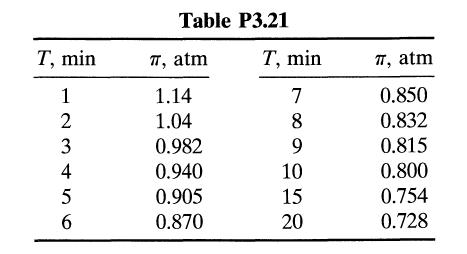
A small reaction bomb fitted with a sensitive pressure-measuring device is flushed out and then filled with pure reactant A at 1-atm pressure. The operation is carried out at 25OC, a temperature low enough that the reaction does not proceed to any appreciable extent. The temperature is then raised as rapidly as possible to 100°C by plunging the bomb into boiling water, and the readings in Table P3.21 are obtained. The stoichiometry of the reaction is 2A → B, and after leaving the bomb in the bath over the weekend the contents are analyzed for A; none can be found. Find a rate equation in units of moles, liters, and minutes which will satisfactorily fit the data.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





