Question: Example 11-1: What Additional Information Is Required? How would this example change if a CSTR were used instead of a PFR? Example 11-1 The first-order
Example 11-1: What Additional Information Is Required? How would this example change if a CSTR were used instead of a PFR?
Example 11-1
The first-order liquid-phase reaction A → B is carried out in a PFR. The reaction is exothermic and the reactor is operated adiabatically. As a result, the temperature will increase with conversion down the length of the reactor. Because T varies along the length of the reactor, k will also vary, which was not the case for isothermal plug-flow reactors.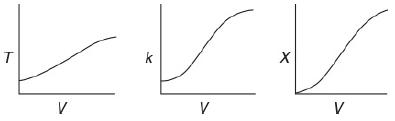
The relationship between V with T, k, and X are graphically portrayed. The first graph compares V and T. The curve begins at T at a short distance above the origin, which increases gradually with increasing V. The second graph compares V and k. The curve begins at k at a short distance above the origin. Initially, the curve is flat and increases gradually with increasing V. The third graph compares V and X. The curve begins at the origin, which increases with increasing V. Describe how to calculate the PFR reactor volume necessary for 70% conversion and plot the corresponding profiles for X and T.
T V k V X V
Step by Step Solution
3.44 Rating (147 Votes )
There are 3 Steps involved in it
Mole balancede... View full answer

Get step-by-step solutions from verified subject matter experts


