Question: Examples 9.4 through 9.7 illustrate the approach to problems dealing with non isothermal reactors. This approach to multistage operations of solid catalyzed reactions. To reinforce
Examples 9.4 through 9.7 illustrate the approach to problems dealing with non isothermal reactors. This approach to multistage operations of solid catalyzed reactions.
To reinforce these concepts, Problems 9.1 through 9.9 ask the reader to redo these examples with one or more changes. In many of these problems there is no need to redo the whole problem, just indicate where changes are needed in text and graphs.
Example 9.4
Using the optimal temperature progression in a plug flow reactor for the reaction
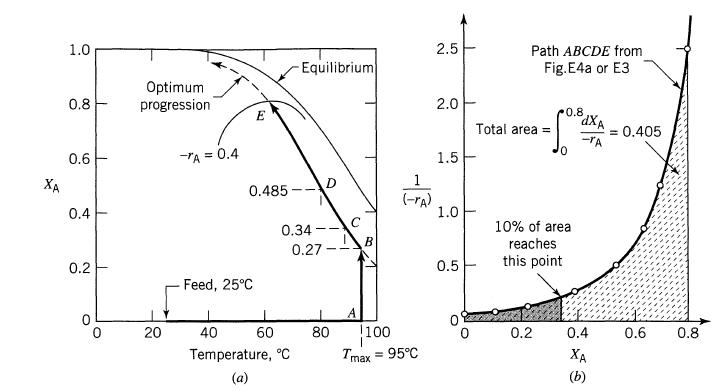
We wish to run the reaction of Example 9.4 in a mixed flow reactor to 95% conversion for a feed concentration CA0 = 10 mollliter and feed rate of v = 100 liter/min. What size of reactor would we need?
XA 1.0 0.8 0.6 0.4 0.2 1 Optimum progression 20 -TA = 0.4 E 0.485 Feed, 25C Equilibrium 0.34- 0.27 40 60 Temperature, C 0 80 C A B (-rA) 1100 I Tmax = 95C 2.5 2.0 1.5 1.0 0.5 0 Path ABCDE from Fig. E4a or E3 Total area = 0.8 0.2 0 10% of area reaches this point dXA -TA 0.4 XA = 0.405 0.6 0.8
Step by Step Solution
3.38 Rating (167 Votes )
There are 3 Steps involved in it
The diagram you sent shows the optimal temperature progression for the reaction A B in a plug flow reactor The xaxis is the temperature C and the yaxi... View full answer

Get step-by-step solutions from verified subject matter experts


