For a gas reaction at 400 K the rate is reported as (a) What are the units
Question:
For a gas reaction at 400 K the rate is reported as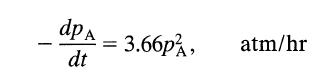
(a) What are the units of the rate constant?
(b) What is the value of the rate constant for this reaction if the rate equation is expressed as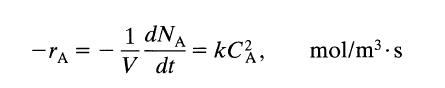
Transcribed Image Text:
dp A dt 3.66p, atm/hr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Hence the value of rate constant is 12013 with units lmolh A gas phase reaction ...View the full answer

Answered By

Umber Talat
I am providing full time mentoring and tutoring services in Business Finance, Contemporary issue in Global Economy, Quantitative Techniques, Principles of Marketing, strategic marketing, International Marketing, Organizational Behavior (OB), Consumer Behavior, Sales Force Management, Strategic Brand Management, Services Marketing, Integrated Marketing Communication (IMC), Principles of Management, General Management, Strategic Management, Small and Medium Enterprise Management, Innovation Management, Change Management, Knowledge Management, Strategic Planning, Operations Management, Supply Chain Management, Logistics Management, Inventory management, Total Quality Management (TQM), Productions Management, Project Management, Production Planning, Human Resource Management (HRM), Human Resource Development, Strategic HRM, Organizational Planning, Performance and Compensation Management, Recruitment and Selection, Organizational Development, Global Issues in Human Resource Management, Retail Marketing, Entrepreneurship, Entrepreneurial Marketing, International Business, Research Methods in Business, Business Communication, Business Ethics.
4.70+
158+ Reviews
236+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
What are component units of a government, and how are they reported on the government-wide financial statements?
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
What are equivalent units of production?
-
Review Questions: 1. What is the theory on which Rockwell hardness testing is based? 2. What is the purpose of the minor load in Rockwell hardness testing? 3. What are the advantages of the Rockwell...
-
1. Explore the Web sites of other companies to learn how they get feedback from customers. Start by clicking on Contact Us, then dig deeply to see if you can find a place where each company seeks or...
-
Prepare Corrected Trail Balance and Journal Entries. The trial balance of the Sterling Company shown below does not balance. STERLING COMPANY Trial Balance May 31, 2010 Accounts Title Debit Credit...
-
25. LO.5 Jayden calculates his 2021 income tax by using both the Tax Tables and the Tax Rate Schedules. Because the Tax Rate Schedules yield a slightly lower tax liability, he plans to pay this...
-
Amy owns a vacation cottage in Maine. She predicts that the time during which the cottage will be used in the current year is as follows: By Amy, solely for vacation and vacationing the rest of the...
-
Pearl Products Limited of Shenzhen, China, manufactures and distributes toys throughout South East Asia. Three cubic centimeters (cc) of solvent H300 are required to manufacture each unit of...
-
The decomposition of nitrous oxide is found to proceed as follows: What is the order of this reaction with respect to N,O, and overall? +21/20 NO N + k[NO] -N0 = 1+ k[NO]
-
A 1100-K n-nonane thermally cracks (breaks down into smaller molecules) 20 times as rapidly as at 1000 K. Find the activation energy for this decomposition.
-
On June 1, 20--, a depreciable asset was acquired for $5,400. The asset has an estimated useful life of five years (60 months) and no salvage value . Using the straight-line depreciation method,...
-
Based on the dangling-else discussion in Exercise 3.27, modify the following code to produce the output shown. Use proper indentation techniques. You must not make any additional changes other than...
-
Consider the random process \(U(t)=A\), where \(A\) is a random variable uniformly distributed on \((-1,1)\). (a) Sketch some sample functions of this process. (b) Find the time autocorrelation...
-
In 2020 the global distribution of sales in the industrial gas industry was as follows: i What is Air Liquides position on a GCI/GRI mapping? Global industrial gas industry 82 billion The 2020 global...
-
The General Social Survey polled a sample of 1048 adults in the year 2010, asking them how many hours per week they spent on the Internet. The sample mean was 9.79 with a standard deviation of 13.41....
-
An article in the Archives of Internal Medicine reported that in a sample of 244 men, 73 had elevated total cholesterol levels (more than 200 milligrams per deciliter). In a sample of 232 women, 44...
-
Form a group of students and research Dominican Republic-Central America-United States (CAFTA-DR) trade agreement for central America. A government website, www.ustr.gov, provides information on...
-
Graph the following conic sections, labeling vertices, foci, directrices, and asymptotes (if they exist). Give the eccentricity of the curve. Use a graphing utility to check your work. 10 5 + 2 cos 0
-
a. Load the ICG on your computer and carry out the exercise. Performance number =________________________. (http://www.umich.edu/~elements/6e/icm/enzyme.html) b. Rederive Equation (9-9) assuming the...
-
Hydrogen radicals are important to sustaining combustion reactions. Consequently, if chemical compounds that can scavenge the hydrogen radicals are introduced, the flames can be extinguished. While...
-
The pyrolysis of acetaldehyde is believed to take place according to the following sequence: CH3CHOk1CH3+CHOCH3+CH3CHOk2CH3+CO+CH4CHO+CH3CHOk3CH3+2CO+H22CH3k4C2H6 a. Derive the rate expression for...
-
Problem 1. Alucard and Chou organized their partnership on 01/01/19. The following entries were made into their capital accounts during 2019. Alucard, capital Chou, capital BB 0 BB 25,000 1/1 35,000...
-
Marshall Inc. Comparative Retained Earnings Statement For the Years Ended December 31, 20Y2 and 20Y1 1 20Y2 20Y1 2 Retained earnings, January 1 $3,716,000.00 $3,266,000.00 3 Net income 630,000.00...
-
S&L Financial buys and sells securities expecting to earn profits on short-term differences in price. On December 27, 2021, S&L purchased Coca-Cola bonds at par for $900,000 and sold the bonds on...

Study smarter with the SolutionInn App


