Pure oxygen is being absorbed by xylene in a catalyzed reaction in the experimental apparatus sketched in
Question:
Pure oxygen is being absorbed by xylene in a catalyzed reaction in the experimental apparatus sketched in Figure P14-3B. Under constant conditions of temperature and liquid composition, the following data were obtained:
In the experiment, the bottom of the apparatus is heated in a trough consists of xylene. The vapor of boiling xylene is collected in a flask, which is observed by pure oxygen, and also some vapor of the xylene goes to the atmosphere while giving some residues of H2O.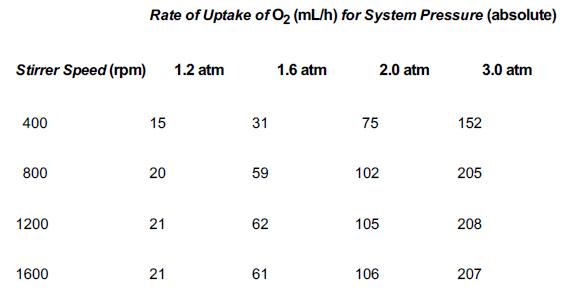
No gaseous products were formed by the chemical reaction. What would you conclude about the relative importance of liquid-phase diffusion and about the order of the kinetics of this reaction?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





