The irreversible reaction A+B C+D is carried out adiabatically in a CSTR. The heat generated G(T)
Question:
The irreversible reaction A+B → C+D is carried out adiabatically in a CSTR. The “heat generated” G(T) and the “heat removed” R(T) curves are shown in Figure P12-14A.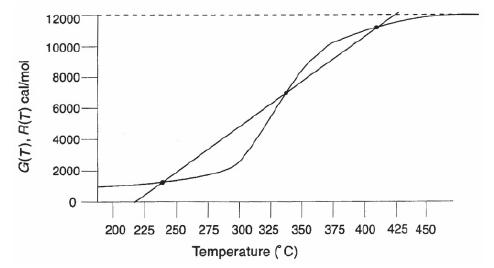
The graph represents the data of heat-removed and heat-generated corresponding to the temperature. The horizontal axis represents the temperature in degree Celsius and ranges from 200 to 450 in increments of 25. The vertical axis represents the values of heat-removed - R(T) and heat-generated - G(T) and ranges from 0 to 12000 in increments of 2000. The heat-removed R(T) is represented in a dotted straight line whereas the heat-generated G(T) is represented as a curve. Both the dotted lines and curve are intertwined and there are steady state points at which the dotted line and the curve intersect each other. The temperature and the respective R(T) and G(T) values at different steady states are as follows: Steady state 1: Temperature - 240 degree Celsius and G(T),R(T) - 1000 cal/mol, Steady state 2: Temperature - 330 degree Celsius and G(T),R(T) - 6400 cal/mol, Steady state 3: Temperature - 410 degree Celsius and G(T),R(T) - 1190 cal/mol. Note that the values
are approximate.
(a) What is the ΔHRx of the reaction?
(b) What are the inlet ignition and extinction temperatures?
(c) What are all the temperatures in the reactor corresponding to the inlet ignition and extinction temperatures?
(d) What are the conversions at the ignition and extinction temperatures?
Step by Step Answer:






