Question: The second-order liquid-phase reaction A B + C is to be carried out isothermally. The entering concentration of A is 1.0 mol/dm 3 .
The second-order liquid-phase reaction A → B + C is to be carried out isothermally. The entering concentration of A is 1.0 mol/dm3. The specific reaction rate is 1.0 dm3/mol=min. A number of used reactors (shown below) are available, each of which has been characterized by an RTD. There are two crimson and white reactors, and three maize and blue reactors available.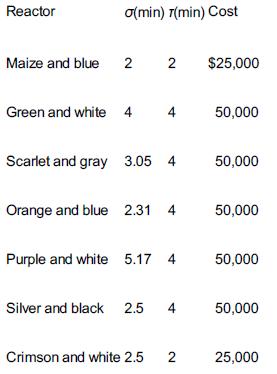
a. You have $50,000 available to spend. What is the greatest conversion you can achieve with the available money and reactors?
b. How would your answer to (a) change if you had an extra $75,000 available to spend?
c. From which cities do you think the various used reactors came from?
Reactor Maize and blue a(min) 7(min) Cost 2 Green and white 4 2 4 Scarlet and gray 3.05 4 Orange and blue 2.31 4 Purple and white 5.17 4 Crimson and white 2.5 Silver and black 2.5 4 2 $25,000 50,000 50,000 50,000 50,000 50,000 25,000
Step by Step Solution
3.38 Rating (154 Votes )
There are 3 Steps involved in it
a The orange blue or silver black reactors which both app... View full answer

Get step-by-step solutions from verified subject matter experts


