The vapor-phase cracking of gas-oil in Example 10-6 is carried out over a different catalyst, for which
Question:
The vapor-phase cracking of gas-oil in Example 10-6 is carried out over a different catalyst, for which the rate law is
−rA′=k′PA2with k′=5×10−5kmolkg-cat⋅s⋅atm2
a. Assuming that you can vary the entering pressure and gas velocity, what operating conditions would you recommend?
b. What could go wrong with the conditions you chose? Now assume the decay law is −dadt=kDaCcoke with kD=100dm3mol⋅Sat 400∘C where the concentration, Ccoke, in mol/dm3, can be determined from a stoichiometric table.
c. For a temperature of 400=C and a reactor height of 15 m, what gas velocity do you recommend? Explain. What is the corresponding conversion?
d. The reaction is now to be carried in an STTR 15 m high and 1.5 m in diameter. The gas velocity is 2.5 m/s. You can operate in the temperature range between 100°C and 500°C. What temperature do you choose, and what is the corresponding conversion?
e. What would the temperature–time trajectory look like for a CSTR?
Additional information: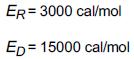
Example 10-6
The vapor-phase cracking of a gas-oil is to be carried out in a straight-through transport reactor (STTR) that is 10-m high and 1.5 m in diameter. Gas-oil is a mixture of normal and branched paraffins (C12–C40), naphthenes, and aromatics, all of which will be lumped as a single species, A. We shall lump the primary hydrocarbon products according to distillate temperature into two respective groups, dry gas (C1–C4) species B and gasoline (C5–C14) species C. The reaction A typical cost of the catalyst in the reactor system is $1 million. Gas oil(g)→Products(g)+Coke can be written symbolically as A→B+C+Coke Both B and C are adsorbed on the surface. The rate law for a gas-oil cracking reaction on fresh catalyst can be approximated by −rA′=k′PA1+KAPA+KBPB+KCPC with k′ = 0.0014 kmol/kg-cat·s·atm, KA = 0.05 atm–1, KB = 0.15 atm–1, and KC = 0.1 atm–1. The catalyst decays by the deposition of coke, which is produced in most cracking reactions along with the reaction products. The decay law is a=11+At1/2with A=7.6 s−1/2 Pure gas-oil enters at a pressure of 12 atm and a temperature of 400=C. The bulk density of catalyst in the STTR is 80 kg-cat/m3. Plot the activity, a(z), and conversion, X(z), of gas-oil up the 10-m reactor for entering gas velocity U0 = 2.5 m/s.
Step by Step Answer:






