With the focus on alternative clean-energy sources, we are moving toward an increased use of fuel cells
Question:
With the focus on alternative clean-energy sources, we are moving toward an increased use of fuel cells to operate appliances ranging
from computers to automobiles. For example, the hydrogen/oxygen fuel cell produces clean energy as the products are water and electricity, which may lead to a hydrogen-based economy instead of a petroleum-based economy. A large component in the processing train for fuel cells is the water-gas shift membrane reactor.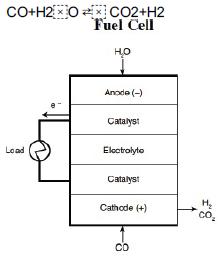
A fuel cell is shown in the form of a rectangle with layers of Anode, Cathode, 2 Catalysts and Electrolyte present in it. The 2 catalysts are connected by a load. The input of Anode is H2O and the input of Cathode is CO. The output of Cathode is hydrogen molecule and carbon dioxide. Here, CO and water are fed to the membrane reactor containing the catalyst. Hydrogen can diffuse out the sides of the membrane, while CO, H2O, and CO2 cannot. Based on the following information, plot the concentrations and molar flow rates of each of the reacting species down the length of the membrane reactor. Assume the following: The volumetric feed is 10 dm3/min at 10 atm, and the equimolar feed of CO and water vapor with CT0 = 0.4 mol/dm3. The equilibrium constant is Ke = 1.44,
with k = 1.37 dm6/mol kg-cat · min, bulk density, ρ = 1000 kg/m3, and the mass transfer coefficient kH2 = 0.1 dm3/kg-cat · min. Hint: First calculate the entering molar flow rate of CO and then relate FA and X.
a. What is the membrane reactor catalyst weight necessary to achieve 85% conversion of CO?
b. Sophia wants you to compare the MR with a conventional PFR. What will you tell her?
c. For that same membrane reactor catalyst weight, Nicolas wants to know what would be the conversion of CO if the feed rate were doubled?
Step by Step Answer:






