Complete and balance each acidbase equation. a. HI(aq) + LiOH(aq) b. HCHO(aq) + Ca(OH)(aq) c. HCl (aq)
Question:
Complete and balance each acid–base equation.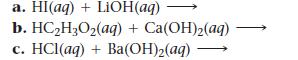
Transcribed Image Text:
a. HI(aq) + LiOH(aq) b. HC₂H₂O₂(aq) + Ca(OH)₂(aq) c. HCl (aq) + Ba(OH)₂(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
Sure here are the completed and balanced a...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Procedures Experiment 1: Standardize the Iodine Solution Part 1: Prepare the Materials Take a 100 mL volumetric flask from the Containers shelf and place it on the workbench. Take ascorbic acid from...
-
Procedures Experiment 1: Standardize the Iodine Solution Part 1: Prepare the Materials Take a 100 mL volumetric flask from the Containers shelf and place it on the workbench. Take ascorbic acid from...
-
First, complete and balance each of the equations below. Then, choosing among ethanol, hexane, and liquid ammonia, state which (there may be more than one) might be suitable solvents for each of...
-
Unlike affirmative action, diversity _ _ _ _ _ . a . can exist even if organizations do not take purposeful steps to create it b . is required by law for private employers with 5 0 or more employees...
-
Why are accountants so concerned about their organization having an efficient and effective internal control system?
-
What are some of the different model-driven methodologies?
-
Do you think that training evaluation must be all or nothing; that is, must it totally determine the worth of training or else not be done at all?
-
The cash account for American Medical Co. at April 30 indicated a balance of $334,985. The bank statement indicated a balance of $388,600 on April 30. Comparing the bank statement and the...
-
Coronado Electronics manufactures two ultra-high-definition television models: the Royale, which sells for $ 1,530, and a new model, the Majestic, which sells for $ 1,320. The production cost...
-
Write a test bench for the elevator controller of Problem 5.10. The test bench has two functions: to simulate the operation of the elevator (including the door operation) and to provide a sequence of...
-
Write balanced complete ionic and net ionic equations for each acidbase reaction. a. HBr(aq) + NaOH(aq) b. HF(aq) + NaOH(aq) C. HCHO(aq) + RbOH(aq)
-
Write balanced molecular and net ionic equations for the reaction between nitric acid and calcium hydroxide.
-
The monthly closing stock price for a large technology firm for the first six months of the year are reported in the following table. Month Price...
-
Arizona Corp. had the following account balances at 12/1/19: Receivables: $96,000; Inventory: $240,000; Land: $720,000; Building: $600,000; Liabilities: $480,000; Common stock: $120,000; Additional...
-
Construct a 90% confidence interval for the population standard deviation o at Bank A. Bank A 4.2 5.4 5.9 6.1 6.6 7.7 7.7 8.6 9.3 10.0
-
Margin of Error For the poll described in Exercise 1, describe what is meant by the statement that "the margin of error was given as +3.5 percentage points."
-
1) Explain what the critical issue was in the Uber decisions in the First Circuit (Culliane case), and the Second Circuit (Myer case), and how each court, looking at the same facts, came to opposite...
-
IFRS LEASE 1. Kappa Berhad enters into a 10-year lease on 1 January 2020. Kappa Berhad incurred the following costs in respect of the lease: RM2,500 legal fees RM15,000 deposit made at the...
-
Consider an 18-8 Mo stainless steel component (Figure 8.35) that is exposed to a temperature of 500C (773 K). What is the maximum allowable stress level for a rupture lifetime of 5 years? 20 years?
-
What are some of the possible sources of information about a company that could be used for determining the companys competitive stance?
-
Calculate the velocity in m/s of a 12-kg object if it has a kinetic energy of 15 Nm. The formula for kinetic energy is KE = mv 2 , where m = mass and v = velocity.
-
Calculate the velocity in m/s of a 175-g body if it has a kinetic energy of 212 m Nm. The formula for kinetic energy is KE = mv 2 , where m = mass and v = velocity.
-
Calculate the kinetic energy in ft-lb of a 1-slug mass if it has a velocity of 4 ft/s. The formula for kinetic energy is KE = mv 2 , where m = mass and v = velocity.
-
do you think regulation helps prevent occurrence of accounting scandals? Or do you think it is positively related to future accounting scandals?
-
Suppose the rate of return on short-term government securities (perceived to be risk-free) is about 5%. Suppose also that the expected rate of return required by the market for a portfolio with a...
-
Year-to-date, Company O had earned a -2.40 percent return. During the same time period, Company V earned 8.3 percent and Company M earned 6.55 percent. If you have a portfolio made up of 10 percent...

Study smarter with the SolutionInn App


