Consider the reactions and their respective equilibrium constants: Use these reactions and their equilibrium constants to predict
Question:
Consider the reactions and their respective equilibrium constants: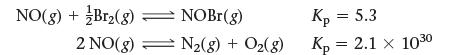
Use these reactions and their equilibrium constants to predict the equilibrium constant for the following reaction:![]()
Transcribed Image Text:
NO(g) + Br₂(g) — NOBr(g) 2 NO(g) N₂(g) N₂(g) + O₂(g) Kp = 5.3 Kp = 2.1 × 1030
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
1...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the reactions and their equilibrium constants to predict the equilibrium constant for the reaction 2 A(s) 3 D(g). A(s) = 3 D(g) B(g) + C(g) B(g) + 2 C(g) K = 0.0334 K = 2.35
-
Consider the following hypothetical reactions. The equilibrium constants K given for each reaction are defined in terms of a concentration unit of molecules per liter. Assume that the reactions have...
-
The equilibrium constant of the dissociation reaction H2 2H at 3000 K and 1 atm is KP1. Express the equilibrium constants of the following reactions at 3000 K in terms of KP1: (a) H, 2H (c) 2H24H (d)...
-
Consider a situation wherein an exploration vehicle that has a mass of 250 kilogram on Earth ( gravityEarth = 9.8 m/s ) is sent to the Moon and planet Mars to explore their surfaces. What is the mass...
-
The following cases are each independent of the others. Required: 1. Lyndon Wilson places $5,000 in a savings account that pays 3 percent. Suppose Lyndon leaves the original deposit plus any interest...
-
Draw a 2inch \(\times\) 2inch square \(O P Q R\) such that \(O P\) makes \(+73^{\circ}\) to the \(x\) axis. Repeat questions (a) through (d) in problem 17 for \(O P Q R\). Give physical...
-
Discuss the concept of earning power, and differentiate it from the concept of solvency. How are they related? How can financial accounting numbers be used to assess each?
-
The egg industry is comprised of many firms producing an identical product. Demand and supply conditions are indicated in the left-hand panel of the figure below; the long run cost curves of a...
-
Question 3 (22 marks) Ivana Company's ("the Company) sales are expected to increase from $10million in 2018 to $12million in 2019. Its assets totaled $6million at the end of 2018. The Company is at...
-
According to the Internal Revenue Service, income tax returns one year averaged $1,332 in refunds for taxpayers. One explanation of this figure is that taxpayers would rather have the government keep...
-
Calculate K c for each reaction. a. [(g) = 21(g) Kp 6.26 x 10-22 (at 298 K) b. CH4(g) + HO(g) = CO(g) + 3 H(g) c. I(g) + Cl(g) = 2 ICI(g) = Kp = 7.7 x 1024 (at 298 K) Kp = 81.9 (at 298 K) P
-
This reaction has an equilibrium constant of Kp = 2.2 * 10 6 at 298 K. Calculate Kp for each reaction and predict whether reactants or products will be favored at equilibrium. 2 COF2(g) = CO(g) +...
-
In Figure 9.2, there is a striped area in the right-hand part of the curve. a. What does that area represent? b. If you tested the research hypothesis at a more rigorous level (say at .01 rather than...
-
j. Interest was accrued on the note receivable received on October 17 ($100,000, 90-day, 9% note). Assume 360 days per year. Date Description Dec. 31 Interest Receivable Interest Revenue Debit Credit
-
A Chief Risk Officer (CRO) is interested in understanding how employees can benefit from AI assistants in a way that reduces risk. How do you respond
-
Based on contract law principles, do you think the jury\'s verdict against the Loewen Group for $ 5 0 0 million was appropriate? Why or why not? What factors should the jury have considered in...
-
5.) Consider you have two systems - one filled with (1kg) water and the other with (1kg) of air. Both systems are at 1000 kPa and 30 C. Determine numerically which fluid system has the larger...
-
Question 3: The partnership of Blossom, Blue, and Kingbird engaged you to adjust its accounting records and convert them uniformly to the accrual basis in anticipation of admitting Kerns as a new...
-
Consider the problem of pricing the following options via PDE: (a) European Call (b) European Put (c) Up-and-Out Call (d) Down-and-Out Put In each case explain reasonable boundary conditions at...
-
In the current year, the City of Omaha donates land worth $500,000 to Ace Corporation to induce it to locate in Omaha and create an estimated 2,000 jobs for its citizens. a. How much income, if any,...
-
An enterprising young man travels to Europe carrying three light bulbs he had purchased in North America. The light bulbs he has are a 100-W light bulb, a 60-W light bulb, and a 40-W light bulb. Each...
-
The 150 W light bulb in Fig. 2.122 is rated at 110 volts. Calculate the value of Vs to make the light bulb operate at its rated conditions. 150 Watt 100 2 +. ww- 50 2
-
Calculate I o in the circuit of Fig. 2.119. 700 2 400 2 200 2 100 V 1.7 k2 800 2 400 2 +1)
-
Your company has preferred stock currently selling for $65.24 on the New York Stock Exchange (NYSE). If the stock paid a dividend of $4.8 last year, what is the cost of preferred stock to your...
-
Rank the following three stocks by their risk-return relationship, best to worst. Rail Haul has an average return of 10 percent and standard deviation of 30 percent. The average return and standard...
-
What is the cash value of a lease requiring payments of $859.00 at the beginning of every six months for 11 years, if interest is 4% compounded quarterly?

Study smarter with the SolutionInn App


