Draw the Lewis structure (including resonance structures) for the acetate ion (CH 3 COO ). For
Question:
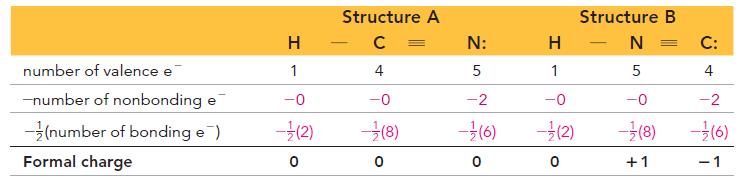
Draw the Lewis structure (including resonance structures) for the acetate ion (CH3COO–). For each resonance structure, assign formal charges to all atoms that have formal charge.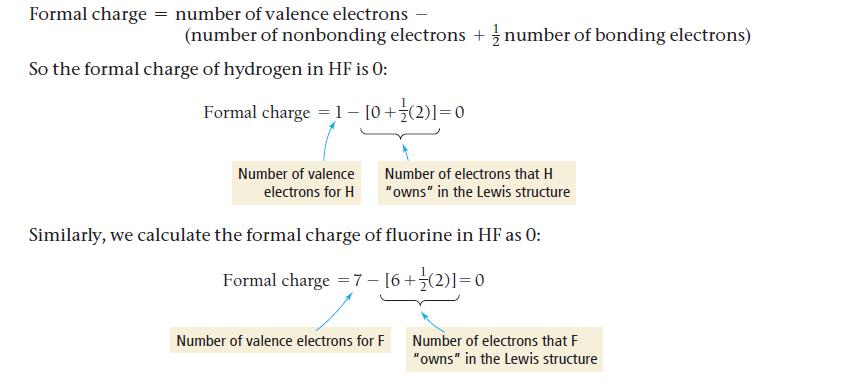
Transcribed Image Text:
Formal charge = number of valence electrons - (number of nonbonding electrons+ number of bonding electrons) So the formal charge of hydrogen in HF is 0: Formal charge = 1- [0+ (2)]=0 Number of valence electrons for H Number of electrons that H "owns" in the Lewis structure Similarly, we calculate the formal charge of fluorine in HF as 0: Formal charge = 7 - [6+ (2)]=0 Number of valence electrons for F Number of electrons that F "owns" in the Lewis structure
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
H HC...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the Lewis structure (including resonance structures) for methyl azide (CH 3 N 3 ). For each resonance structure, assign formal charges to all atoms that have formal charge. Formal charge number...
-
Briefly describe some common information system controls that need to be implemented by business managers, not IS professionals.
-
What are the differences between a data entity in structured techniques and an object in object-oriented techniques?
-
5.8 Calculate the U value for the following double- glazed windows assuming the temperatures and the heat transfer coefficients as given in Example 5.1: (a) Ordinary glass with vacuum between the...
-
What are object-oriented databases? What are multimedia databases? How are these two types of databases alike? How are they different?
-
Indicate whether each of the following statements is true or false by writing T or F i n t he a nswer c olumn. When threats are used to force someone to enter into a contract, the agreement may be...
-
Customer profitability, service company. Instant Service (IS) is a repair service company specializing in the rapid repair of photocopying machines. Each of its ten clients pays a fixed monthly...
-
The registration area has just opened at a large convention of building contractors in Las Vegas. There are 200 people arriving per hour (Poisson distributed), and the cost of their waiting time in...
-
Special Order Decisions Weber Company produces and sells 10,000 gallons of carrot juice per month. The maximum number of gallons that can be produced per month is 12,000 gallons. Kroger has offered...
-
On May 1, 2024, Christina Fashions borrowed $100,000 at a bank by signing a four-year, 6% loan. The terms of the loan require equal principal payments of $25,000 and accrued interest at 6% due...
-
What are the formal charges of the atoms shown in red? CH3 CH3N0: -N- CH3
-
How important is the resonance structure shown here to the overall structure of carbon dioxide? Explain. :0=C:
-
(1) Let \(M\) be a positive continuous martingale such that \(M_{0}=x\). (i) Prove that if \(\lim _{t ightarrow \infty} M_{t}=0\), then \[\begin{equation*}\mathbb{P}\left(\sup M_{t}>a...
-
Sketch a low-power Schottky TTLNAND circuit. What are the primary differences between this circuit and the regular DTL circuit considered earlier in the chapter?
-
The following situation happened to a friend of mine. One weekend she was leaving to go to a beach resort with her husband. Because she was short of money, she asked her husband to stop at an ATM...
-
In free space, Maxwell's equations simplify greatly. The two equations involving surface integrals of the fields (Eqs. 30. 10 and 30. 11) are zero, and the two equations involving line integrals of...
-
If in Figure P32.33 the two gates on the left were both AND and the gate on the right was OR, what combinations of positive bias (input signals) A, B, C, D would allow the light bulb to light up?...
-
Why are none of the bulbs in Figure 31. 24 lit? Data from Figure 31. 24 Figure 31.24 (1) (iii) NNN
-
Calculate the moduli of resilience for the materials having the stress-strain behaviors shown in Figures 6.12 and 6.21.
-
The Alert Company is a closely held investment-services group that has been very successful over the past five years, consistently providing most members of the top management group with 50% bonuses....
-
Figure 3.33 shows a manometer being used to indicate the difference in pressure between two points in a pipe. Calculate (p A - p B ). Oil (sg = 0.90) 3 ft 2'ft 6 ft Water
-
For the well-type manometer in Fig. 3.34, calculate p A . 6,8 in PA Water
-
Figure 3.35 shows an inclined well-type manometer in which the distance L indicates the movement of the gage fluid level as the pressure pA is applied above the well. The gage fluid has a specific...
-
(Present value)Sarah Wiggum would like to make a single investment and have $2.2 million at the time of her retirement in 35 years. She has found a mutual fund that will earn 7 percent annually. How...
-
Fraudulent financial reporting is an intentional misstatement or omission of amounts or disclosures with the intent to deceive users. Select one: True False
-
I am having trouble solving this problem on question #27. Can you please show me how to solve it step by step? Cost Mastery Problemi cost-Volume-Profit Analysis Cost Behavior Cover-to-Cover Company...

Study smarter with the SolutionInn App


