Evidence for the additional stabilization of certain electron configurations comes from the experimental lattice energies of the
Question:
Evidence for the additional stabilization of certain electron configurations comes from the experimental lattice energies of the metal fluorides, MF2. The first figure below plots lattice energy for the 2+ metal cations for period 4 elements. The figure that follows plots the ionic radii of the 2+ metal cations of period 4 elements versus atomic number.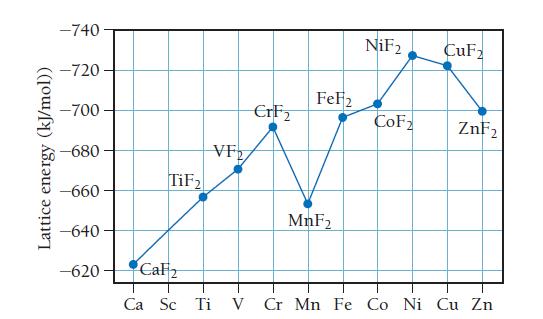
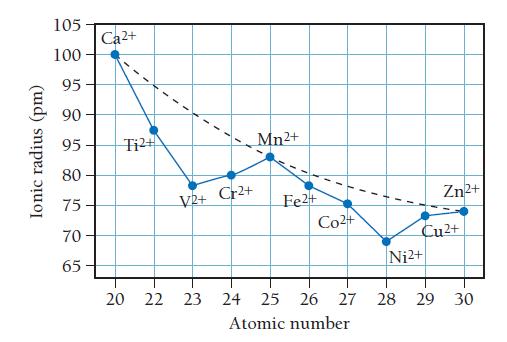
Use the information provided in the figures to answer the following questions:
a. Explain the general trend in lattice energy.
b. Is there a correlation between ionic radius and lattice energy? Explain.
c. What could account for the decrease in lattice energy between CrF2 and MnF2?
d. Which has the higher lattice energy: VF2 or VCl2 ? Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





