For each reaction, calculate H rxn , S rxn , and G rxn at 25 C and
Question:
For each reaction, calculate ΔH°rxn, ΔS°rxn, and ΔG°rxn at 25 °C and state whether or not the reaction is spontaneous. If the reaction is not spontaneous, would a change in temperature make it spontaneous? If so, should the temperature be raised or lowered from 25 °C?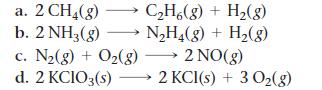
Transcribed Image Text:
a. 2 CH₂(g) b. 2 NH3(g) C₂H6(g) + H₂(g) N₂H₂(g) + H₂(g) c. N₂(g) + O₂(g) →→→ 2 NO(g) d. 2 KCIO3(s)→→→→→2 KCl(s) + 3 O₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To calculate Hrxn Srxn and Grxn we need the standard enthalpy of formation Hf values and standard en...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suppose that your company has decided to invest RM316 million in a 5-year construction project. The project is expected to be sold for RM200 million once completed. Estimate the Internal Rate of...
-
Use standard free energies of formation to calculate G at 25 C for each reaction in Problem 62. How well do the values of G calculated this way compare to those calculated from H and S? Which of the...
-
Use standard free energies of formation to calculate G at 25 C for each reaction in Problem 61. How do the values of G calculated this way compare to those calculated from H and S? Which of the two...
-
Why is it important to have a defined project scope? Why is it important to make sure there is agreement about the scope? Is there anything in the "Why Should You Use the WBS?
-
Discuss the distinguishing features of the Japanese distribution system.
-
The bookkeeper at Tony Company has asked you to prepare a bank reconciliation as of February 29. The February bank statement and the February T- account for cash showed the following (summarized):...
-
Claim: p 0.04; =
-
A heat-treated steel shaft is to be designed to support the spur gear and the overhanging worm shown in the figure. A bearing at A takes pure radial load. The bearing at B takes the worm-thrust load...
-
4) When a firm increases its debt ratio a) its weighted average cost of capital increases b) its cost of debt and cost of equity increase c) both a and b are true d) its weighted average cost of...
-
Determine G for the reaction: Use the following reactions with known G rxn values: FeO3(s) + 3 CO(g) 2 Fe(s) + 3 CO(g)
-
In photosynthesis, plants form glucose (C 6 H 12 O 6 ) and oxygen from carbon dioxide and water. Write a balanced equation for photosynthesis and calculate H rxn , S rxn , and G rxn at 25 C. Is...
-
Refer to Exhibit 2.3 and briefly describe the frauds that were perpetrated at the following companies. For each company, categorize the fraud as involving primarily (1) asset misappropriation, or (2)...
-
Photon Technologies, Inc., a manufacturer of batteries for mobile phones, signed a contract with a large electronics manufacturer to produce three models of lithium-ion battery packs for a new line...
-
Mastery Problem: Capital Investment Analysis HomeGrown Company HomeGrown Company is a chain of grocery stores that are similar to indoor farmer's markets, providing fresh, local produce, meats, and...
-
McDonald's and CSR There more than 32,000 restaurants around the world (www.aboutmcdonalds.com/etc/medialib/csr/docs. that carry the McDonald's label and logo. As such, they...
-
Smartwatch Based on a survey by Consumer Technology Association, smartwatches are used in 18% of U.S. households. Find the probability that a randomly selected U.S. household has no smartwatches.
-
Suppose you wanted to purchase a commercial real estate property thats valued at $ 1 , 0 0 0 , 0 0 0 . You could secure financing from a traditional bank, which provides you with $ 7 5 0 , 0 0 0 ....
-
Wal-Mart Stores, Inc., is the world's largest retailer. A large portion of the premises that the company occupies are leased. Its financial statements and disclosure notes revealed the following...
-
A glass manufacturer produces hand mirrors. Each mirror is supposed to meet company standards for such things as glass thickness, ability to reflect, size of handle, quality of glass, color of...
-
Consider the flagpole in Figure P8.29. If the flagpole has a mass of 20 kg and length 10 m and the angle the cable makes with the pole is ?? = 25, what are the magnitude and direction of the force...
-
A painter is standing on the ladder (mass 40 kg and length 2.5 m) in Figure P8.30. There is friction between the bottom of the ladder and the floor with S = 0.30, but there is no friction between...
-
Consider again the ladder in Problem 30. What is the sign of the torque on the ladder due to the force from the wall? Data from Problem 30 A painter is standing on the ladder (mass 40 kg and length...
-
Question 4. - Week 9. What are the major competitive issues General Electric faces when managing cooperative strategies? - (7 marks)
-
All of the following are roles of a derivative exchange EXCEPT: _____. A) maintaining margin requirements on futures contracts B) reducing the default risk on forward contracts C) performing daily...
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...

Study smarter with the SolutionInn App


