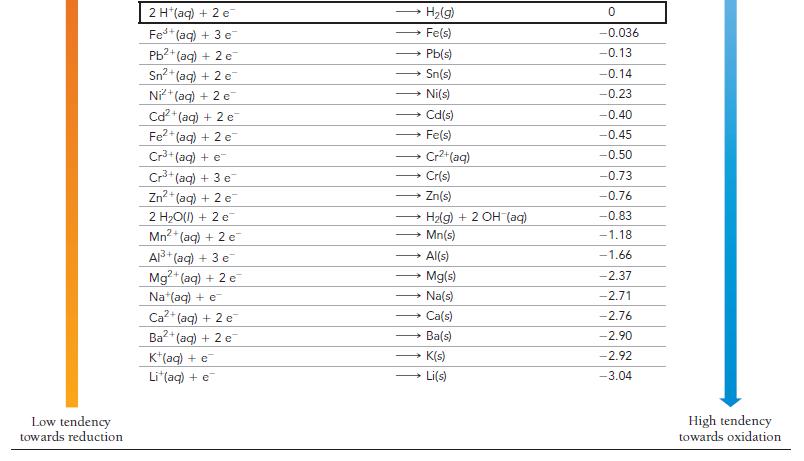
How can Table 20.1 be used to predict whether or not a metal will dissolve in HCl?
Question:
How can Table 20.1 be used to predict whether or not a metal will dissolve in HCl? In HNO3?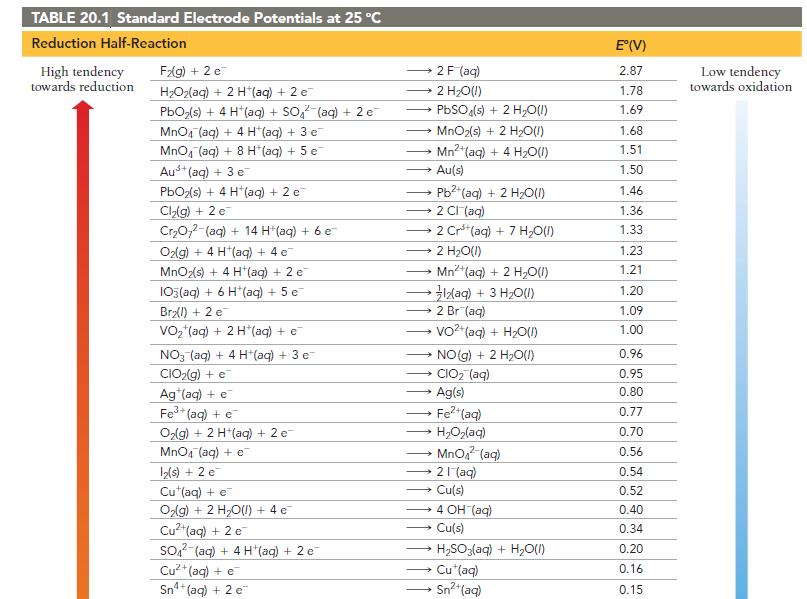
Transcribed Image Text:
TABLE 20.1 Standard Electrode Potentials at 25 °C Reduction Half-Reaction High tendency towards reduction F₂(g) + 2 e H₂O₂(aq) + 2 H+(aq) + 2 e PbO₂ (s) + 4 H*(aq) + SO² (aq) + 2 e MnO4 (aq) + 4 H (aq) + 3 e MnO4 (aq) + 8 H(aq) + 5 e Aus+ (aq) + 3 e PbO₂(s) + 4 H*(aq) + 2 e Cl₂(g) + 2 e Cr₂O72-(aq) + 14 H+ (aq) + 6 e- O₂(g) + 4 H (aq) + 4 e MnO₂(s) + 4 H*(aq) + 2 e 103(aq) + 6 H*(aq) + 5 e Br₂(l) + 2 e VO₂ (aq) + 2 H*(aq) + €¯ NO3(aq) + 4 H+(aq) + 3 e CIO₂(g) + e Ag (aq) + e Fe³+ (aq) + e O₂(g) + 2 H+(aq) + 2 e- MnO4 (aq) + e 12(s) + 2 e™ Cu (aq) + e O₂(g) + 2 H₂O(l) + 4e¯ Cu²+ (aq) + 2 e SO2 (aq) + 4 H(aq) + 2 e Cu²+ (aq) + e Sn²4+ (aq) + 2 c → Au(s) 2 F (aq) 2 H₂O(l) PbSO4(s) + 2 H₂O(l) MnO2(s) + 2 H₂O(l) Mn²+ (aq) + 4H₂O(1) → 2 CI (aq) - Pb2+ (aq) + 2 H₂O(l) - 2 Cr³+ (aq) + 7 H₂O(l) 2 H₂O(1) Mn²+ (aq) + 2 H₂O(l) • }zaq) + 3 HO(I) 2 Br (aq) VO²+ (aq) + H₂O(l) → CIO₂ (aq) Ag(s) Fe²+ (aq) HyOzlag) MnO₂ (aq) 21 (aq) → NO(g) + 2 H₂O(1) → Cu(s) → 4 OH (aq) Cu(s) H₂SO3(aq) + H₂O(l) Cu*(aq) Sn²+ (aq) E°(V) 2.87 1.78 1.69 1.68 1.51 1.50 1.46 1.36 1.33 1.23 1.21 1.20 1.09 1.00 0.96 0.95 0.80 0.77 0.70 0.56 0.54 0.52 0.40 0.34 0.20 0.16 0.15 Low tendency towards oxidation
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Table 201 titled Standard Electrode Potentials at 25C can be used to predict whether a metal will di...View the full answer

Answered By

Amos Kiprotich
I am a wild researcher and I guarantee you a well written paper that is plagiarism free. I am a good time manager and hence you are assured that your paper will always be delivered a head of time. My services are cheap and the prices include a series of revisions, free referencing and formatting.
4.90+
15+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A version of simple exponential smoothing can be used to predict the outcome of sporting events. To illustrate, consider pro football. Assume for simplicity that all games are played on a neutral...
-
Every year, millions of high school students apply and vie for acceptance to a college of their choice. For many students and their parents, this requires years of preparation, especially for those...
-
An online retailer is offering a new line of running shoes. The retailer plans to send out an e-mail with a discount offer to some of its existing customers and wants to know if it can use data...
-
PROJECT SUMMARY: You have been asked to submit a proposal to a client, Sara Johnson, who is moving the small firm to a new office location. The proposal is on the analysis and design of the office...
-
Reuse Cookware, Inc., manufactures sets of heavy-duty pots. It has just completed production for August. At the beginning of August, its Work in process Inventory account showed direct materials...
-
What are the three major types of negotiated remedies and how do they differ?
-
16. Consider the last rowof Table 1. What is the solution for S and S when ks = kr = 0? (This answer does not require calculation.) In the following five problems, assume that the spot price of gold...
-
Harvard University has recently revolutionized its financial aid policies, aimed at easing the financial strain on middle and upper-middle income families (Newsweek, August 1825, 2008). The expected...
-
Hominy, Inc., has debt outstanding with a face value of $5 million. The value of the firm if it were entirely financed by equity would be $18.15 million. The company also has 420,000 shares of stock...
-
Which metal can be used as a sacrificial electrode to prevent the rusting of an iron pipe? a) Au b) Ag c) Cu d) Mn
-
Does a large positive electrode potential indicate a strong oxidizing agent or a strong reducing agent? What about a large negative electrode potential?
-
Why is treated water sprayed into the air before it is piped to users?
-
A company determines that monthly sales S(t), in thousands of dollars, after t months of marketing a product is given by S(t) = 23-551 + 230t+ 160. a) Find S'(1), S'(2), and S'(4). b) Find S''(1),...
-
Dan is a 16 year-old who decided to skip his adolescent development class. If Dan was 19 years-old, this would be his choice, but because of his age, he has broken the law. What type of offence did...
-
You need to remove a bolt from a metal door. The maximum torque the bolt can withstand before starting to rotate is 7 = 70 N-m. You apply a wrench of m = 0.5 kg and 1 = 0.3 m long. You push down on...
-
Lesson 10.1: Emotional Intelligence Emotional Intelligence is a type of social intelligence that affords the individual the ability to monitor his own and others' emotions, to discriminate among...
-
Harriet??s annuity has a total cash value of $2000, and she has paid a total of $1,500 into it. Under a Section 1035 exchange, Harriet rolls the entire value of the annuity into a different annuity....
-
Alexi Co, issued $25 million face amount of 6%, 10-year bonds on June 1. 2016. The bonds pay interest on an annual basis on May 31 each year. Required: a. Assume that the market interest rates were...
-
I frequently use NY Times and CNN and am aware of Fox News but I never use it. I visit these sites, NY Times and CNN, a few times a week whenever I have to research something or see something on...
-
Blood flows through an artery of diameter d and length L, and the pressure difference between the ends of the artery is P. If the diameter is reduced by a factor of two and the pressure difference is...
-
What will happen if water is poured on top of the mercury and ball bearing of Problem 78 such that the ball bearing is completely submerged in the water? Will the ball bearing (a) sink farther into...
-
A small, square plank of oak floats in a beaker half full of water. The piece of oak is 6.0 cm on a side and 3.0 cm thick and floats on its side as shown in Figure P10.80. (a) Find the location of...
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


