Nitromethane (CH 3 NO 2 ) burns in air to produce significant amounts of heat. How much
Question:
Nitromethane (CH3NO2) burns in air to produce significant amounts of heat.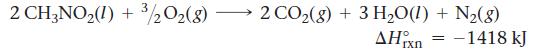
How much heat is produced by the complete reaction of 5.56 kg of nitromethane?
Transcribed Image Text:
2 CH3NO2(I) + 3/2O2(g) 2 CO2(g) + 3 H2O(1) + N2(g) rxn = –1418 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
64...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Magnesium burns in air to produce magnesium oxide, MgO, and magnesium nitride, Mg 3 N 2 . Magnesium nitride reacts with water to give ammonia. Mg 3 N 2 (s) + 6H 2 O(l) 3Mg(OH) 2 (s) + 2NH 3 (g) What...
-
In addition to each process having unique fixed costs, each process also has unique variable costs. In other words, the processing cost per unit is different for each process. Process variation is a...
-
Disk A, with a mass of 2.0 kg and a radius of 50. cm, rotates clockwise about a frictionless vertical axle at 30. rev/s. Disk B, 1.0 kg with a radius of 100. cm, rotates counterclockwise at 20. rev/s...
-
Make a comparison of the following insurance coverage premium bids and determine from a costing standpoint, which offer is better. Show your calculations: Assume that you, as a risk manager would...
-
The internal control procedures in Payton Company make the following provisions. Identify the principles of internal control that are being followed in each case. (a) Employees who have physical...
-
Critically evaluate the argument that an independent central bank should be conservative but not too conservative.
-
What is additive bilingualism?
-
Presented below are selected account balances for Alistair Co. as of December 31, 2012. InstructionsPrepare closing entries for Alistair Co. on December 31, 2012. (Omitexplanations.) Inventory...
-
Pardee company plans to sell 12,000 units during the month of August. If the company will have 3,500 units on hand at the start of the next month and plans to have 2,000 units at the beginning of...
-
1. Case Exhibit 2 presents monthly data of units produced and sold, and actual costs incurred, for 24 months. B Create a scatterplot of costs and units. b. From your scatterplot, estimate the...
-
Titanium reacts with iodine to form titanium(III) iodide, emitting heat. Determine the masses of titanium and iodine that react if 1.55 * 10 3 kJ of heat is emitted by the reaction. 2 Ti(s) + 3 1(g)...
-
What mass of natural gas (CH4) must burn to emit 267 kJ of heat? CH4(g) + 2 Oz(g) COz(g) + 2H,O(g) AH = -802.3 kJ xxn
-
A digital filter is characterized by the following properties: (1) It is high pass and has one pole and one zero. (2) The pole is at a distance r = 0.9 from the origin of the z -plane. (3) Constant...
-
"The initial speed with which a ball is thrown is doubled, with the angle of projection fixed. Is the maximum height to which the ball rises doubled?" Now, let's say you are also allowed to change...
-
Wally Working Co. emiti bonos con una tasa de inters nominal (contratada) de 15%, por un valor ominal de $80,000, con un vencimiento de 5 anios. Cuando emiti los bonos, la tasa de inters del mercado...
-
Using the Central Limit Theorem. In Exercises 5-8, assume that the amounts of weight that male college students gain during their freshman year are normally distributed with a mean of 1.2 kg and a...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
Please help Calculating NPV and IRR Businesses use NPV and IRR to determine whether a project will add - value for shareholders. After watching the CFA Level I Corporate Finance video, answer the...
-
The probability of an electronic sensor malfunctioning is known to be 0.10. A random sample of 12 sensors is chosen. Find the probability that: (a) At least 3 will malfunction. (b) No more than 5...
-
A company has the following incomplete production budget data for the first quarter: In the previous December, ending inventory was 200 units, which was the minimum required, at 10% of projected...
-
One classic problem in quantum mechanics is the harmonic oscillator. In this problem a particle is subjected to a one-dimensional potential (taken to be along x) of the form V (x) x 2 , where x ....
-
A crude model for the molecular distribution of atmospheric gases above the Earths surface (denoted by height, h) can be obtained by considering the potential energy due to gravity: In this...
-
Another use of distribution functions is determining the most-probable value, which is done by realizing that at the distribution maximum the derivative of the distribution function with respect to...
-
ABC Corp currently has a debt to enterprise value ratio of 51%. The firm's cost of equity is 8.4% and its cost of debt is 4%. Assuming perfect markets, calculate the unlevered cost of capital for ABC...
-
Rebound Airlines was hurt in the recession, with its stock falling to $10 from $50. The stock has started to recover and has just crossed its 50-day moving average at $20. The 200-day moving average...
-
Biotech Corp has no sales or earnings but is working on a COVID cure. They also have some good prospects for other drugs that could potentially be blockbusters. The stock started the year at $30 and...

Study smarter with the SolutionInn App


