What volume (in mL) of a soft drink that is 10.5% sucrose (C 12 H 22 O
Question:
What volume (in mL) of a soft drink that is 10.5% sucrose (C12H22O11) by mass contains 78.5 g of sucrose?
(The density of the solution is 1.04 g/mL.)
Transcribed Image Text:
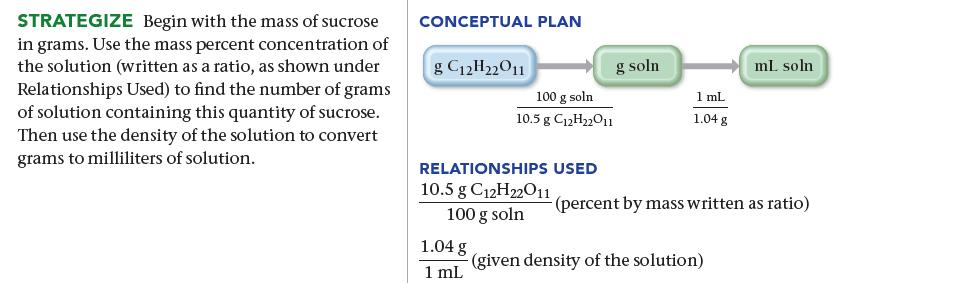
SORT You are given a mass of sucrose and the concentration and density of a sucrose solution, and you are asked to find the volume of solution containing the given mass. GIVEN: 78.5 g C12H22O11 10.5% C12H22011 by mass density = 1.04 g/mL FIND: ML
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
785 g C12H220...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Experiment 2: Osmosis - Direction and ConcentrationGradients In this experiment, we will investigate the effect of soluteconcentration on osmosis. A semi-permeable membrane (dialysistubing) and...
-
The amount of a soft drink that goes into a typical 12-ounce can varies from can to can. It is normally distributed with an adjustable mean and a fixed standard deviation of 0.05 ounce. a. If...
-
Calculate the vapor pressure at 25 C of a solution containing 99.5 g sucrose (C 12 H 22 O 11 ) and 300.0 mL water. The vapor pressure of pure water at 25 C is 23.8 torr. Assume the density of water...
-
Briefly explain the DHCP lease process. What packets are sent and when are they sent? (5 marks) Question 2 any three advantages of IPv6 over IPv4? How many classes are there in IPv4 and what is the...
-
What is customer value? How is customer value related to a cost leadership strategy? To a differentiation strategy? To strategic positioning?
-
The following open-loop continuous-time transfer function represents a second-order system that is to be controlled using a digital computer with \(\mathrm{ZOH}\) : \[G(s)=\frac{1}{(s+1)(s+10)}\]...
-
In 1990 General Motors recorded a $2.1 billion special charge to cover plant closings. In fact, the companys chief financial officer commented in The Wall Street Journal (November 11, 1990) that the...
-
Cost data for F. Mills Manufacturing Company for the month ending April 30, 2010, are as follows: a. Prepare a cost of goods manufactured statement for April 2010. b. Determine the cost of goods sold...
-
Teds is highly risk averse, while Sonny Outlook actually enjoys taking a risk. Investments Buy stocks Buy bonds Buy condities Buy options Returns: Expected Value $ 9,470 7,560 20,400 18,800 Standard...
-
Gibson Agency Case: 1. Calculate and present the budgeted profit for each of Gibson's clients for each of the years 2016 through 2019, using the current costing system (i.e., the one described in the...
-
What does it mean to say that a substance is soluble in another substance? Which units are used in reporting solubility?
-
A 500.0-mL sample of pure water is allowed to come to equilibrium with pure oxygen gas at a pressure of 755 mmHg. What mass of oxygen gas dissolves in the water? (The Henrys law constant for oxygen...
-
Do you want to try your hand at producing a podcast? Businesses rely on a host of social media and communication technologies when reaching out to the public or internally to their workers. As you...
-
On December 1, Year 1, Wayne and Susan Li formed a corporation called French Broad Equipment Rentals. The new corporation was able to begin operations immediately by purchasing the assets and taking...
-
a street light is at the top of a 25 ft pole. A 5 ft girl walks along a straight path away from the pole with a speed of 3 ft/sec. At what rate is the tip of the shadow moving away from the light...
-
Explain why its important to study management.
-
Wildhorse has not logged since 2016. If Wildhorse logged and sold 1,062,000 board feet of timber in 2027, when the timber cruise (appraiser) estimated 5,900,000 board feet, determine the cost of...
-
Y = AK[1-a R P = QAKa-1[1-a W P = (1 -Q) AKL-a 1= 14 1 -4 Y = C
-
Following the derivation of price of default able bond for hazard rate approach, find the expression for default able bond assuming that underlying process follows the CGMY process. The Lévy...
-
(a) Use integration by parts to show that (b) If f and g are inverse functions and f' is continuous, prove that (c) In the case where f and t are positive functions and b > a > 0, draw a diagram to...
-
A standard 6-in Schedule 40 steel pipe is carrying 95 gal/min of water. The pipe then branches into two standard 3-in pipes. If the flow divides evenly between the branches, calculate the velocity of...
-
Repeat Problem 6.51 for a 4-in Schedule 80 pipe. Repeat Problem Compute the resulting velocity of flow if 400 gal/min of fluid flows through a 4-in Schedule 40 pipe.
-
Compute the resulting velocity of flow if 400 gal/min of fluid flows through a 4-in Schedule 40 pipe.
-
Your company has purchased equipment worth $40,000 and would like to compare the impact of straight-line depreciation versus accelerated depreciation (double declining method). The equipment has a...
-
You have $22,000 to invest. You want to purchase shares of Alaska Air at $43.76, Best Buy at $52.62, and Ford Motor at $9.16. How many shares of each company should you purchase so that your...
-
Q 1: (A) What are open market operations? How do these work as a method of credit control? (B): What is the likely impact of money creation by the commercial banks on national income? explain briefly...

Study smarter with the SolutionInn App


