Assign oxidation numbers to all the atoms in each of the following: a. HNO3 b. CuCl c.
Question:
Assign oxidation numbers to all the atoms in each of the following:
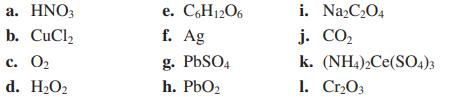
Transcribed Image Text:
a. HNO3 b. CuCl₂ c. 0₂ d. H₂O₂ e. C6H12O6 f. Ag g. PbSO4 h. PbO₂ i. Na₂C₂O4 j. CO₂ k. (NH4)2Ce(SO4)3 1. Cr₂03
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The oxidation numbers of all the atoms in each of the following compounds are a HNO3 H1 N5 O2 b CuCl ...View the full answer

Answered By

Ann Wangechi
hey, there, paying attention to detail is one of my strong points, i do my very best combined with passion. i enjoy researching since the net is one of my favorite places to be and to learn. i am a proficient and versatile blog, article academic and research writing i possess excellent English writing skills, great proof-reading. i am a good communicator and always provide feedback in real time. i'm experienced in the writing field, competent in computing, essays, accounting and research work and also as a Database and Systems Administrator
4.90+
151+ Reviews
291+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Assign oxidation numbers to all the atoms in each of the following: a. HNO 3 b. CuCl 2 c. O 2 d. H 2 O 2 e. C 6 H 12 O 6 f. Ag g. PbSO 4 h. PbO 2 i. Na 2 C 2 O 4 j. CO 2 k. (NH 4 ) 2 Ce(SO 4 ) 3 l....
-
Assign oxidation numbers to the atoms in each substance. a. Kr (krypton) b. krypton tetrafluoride (KrF4) c. dioxygen difluoride (O2F2)
-
Assign oxidation numbers to the atoms in each substance. a. Lithium hydride (LiH) b. Potassium peroxide (K2O2) c. Potassium fluoride (KF)
-
I recently heard my neighbor discussing how one of our other neighbors lost his job. My neighbor assumed that the person who lost his job was probably lazy or not smart enough for the job, without...
-
Prepare the journal entries to record these transactions on Kesler Companys books using a periodic inventory system. (a) On March 2, Kesler Company purchased $800,000 of merchandise from Rice...
-
What are the primary reasons for most legal malpractice claims?
-
*25.5 A company's income statement for the year to 31 March 2010 is as follows: Sales 254,628 Less: Cost of sales 112,876 Gross profit 141,752 Add: Profit on sale of tangible fixed asset 542 Other...
-
Don Wallss gross earnings for the week were $1,780, his federal income tax withholding was $301.63, and his FICA total was $135.73. Instructions (a) What was Wallss net pay for the week? (b)...
-
Below is selected financial information from The Kroger Co.'s FY 2018 10-K. Kroger values its inventory using LIFO. Below you are asked to adjust the balance sheet for a hypothetical conversion to...
-
WestGas Conveyance, Inc. WestGas Conveyance, Inc., is a large U.S. natural gas pipeline company that wants to raise $120 million to finance expansion. WestGas wants a capital structure that is 50%...
-
In the electrolysis of an aqueous solution of Na 2 SO 4 , what reactions occur at the anode and the cathode (assuming standard conditions)? 2- S0 +2e 250 O + 4H+ + 4e 2HO 2HO + 2e H + 2OH Na + e Na...
-
(a) How is resistance present in all transmission lines? (b) How is inductance present in all transmission lines? (c) How is capacitance present in all transmission lines? (d) The combined effects of...
-
Refi Corporation is planning to repurchase part of its common stock by issuing corporate debt. As a result, the firms debt-equity ratio is expected to rise from 35 percent to 50 percent. The firm...
-
7. Chicago Corp. obtained the following information from the Raw Materials Inventory account and purchasing records for the first quarter of the current year: Beginning Raw Materials Ending Raw...
-
Suppose that i t =6% (n=1), and that future short term interest rates (n=1) for the next 3 years (starting next year) are expected to be: 4%, 2%, 2%. Suppose that the liquidity premium is zero for...
-
Mechanical Vibrations HW Use the modal analysis and numerical integration to compute and plot the time response of the system, which has the equations of motion [8 0 01 (1) 48 -12 01(x1 0 0 8 02-12...
-
Submit excel file with graph and exchange rate analysis. FOREIGN EXCHANGE RATESTHE YEN FOR DOLLARS. The Federal Reserve System Web site, www.federalreserve.gov/releases/H10/hist , provides historical...
-
Part 1: There are many types of communication styles used in the workplace. Choose what you think is your leadership style: north, south, east, or west. Click The Leadership Compass Self-Assessment...
-
Name the main elements of the GSM system architecture and describe their functions. What are the advantages of specifying not only the radio interface but also all internal interfaces of the GSM...
-
Find the reduced echelon form of each of the matrices given in Problems 120. c 1 26 + 4
-
Can a molecule with an inversion center have a dipole moment? Give an example of a molecule with this symmetry element and explain your reasoning.
-
Which of the three normal modes of H 2 O in Figure 27.9 is best described as a bending mode? Does the bond angle remain unchanged in any of the modes? Which requires less energy, bond bending or bond...
-
Why does the list of elements for the D 6h group in Table 27.2 not list the elements C 2 6 , C 3 6 , and C 4 6 ? Selected Point Groups and Their Elements Symmetry Elements TABLE 27.2 Example Molecule...
-
Use the following information for questions 2 and 3. Niles Co. has the following data related to an item of inventory: Inventory, March 1 100 units @ $4.20 Purchase, March 7 350 units @ $4.40...
-
Rotan, Inc. purchased a van on January 1, 2018, for $800,000. Estimated life of the van was five years, and its estimated residual value was $96,000. Rotan uses the straightline method of...
-
If at the beginning of a period, you buy a share of stock for $49, then receive a dividend of $3, and finally sell the stock for $51, what was your holding period return? 9.3% 10.2% 14.8% 16.3%

Study smarter with the SolutionInn App


