Calculate the value of the equilibrium constant for each of the following reactions in aqueous solution. a.
Question:
Calculate the value of the equilibrium constant for each of the following reactions in aqueous solution.
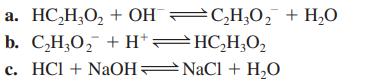
Transcribed Image Text:
a.
HC₂H₂O₂ + OHC₂H₂O₂ + H₂O
b. C,H,O, +H*
a.
HC₂H₂O₂ + OHC₂H₂O₂ + H₂O
b. C,H,O, +H*
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
Image a chemical equation for a chemical reaction HC2H2O2 OH C2H2O2 H2O The equil...View the full answer

Answered By

Cyrus Sandoval
I a web and systems developer with a vast array of knowledge in many different front end and back end languages, responsive frameworks, databases, and best code practices. My objective is simply to be the best web developer that i can be and to contribute to the technology industry all that i know and i can do. My skills include:
- Front end languages: css, HTML, Javascript, XML
- Frameworks: Angular, Jquery, Bootstrap, Jasmine, Mocha
- Back End Languages: Java, Javascript, PHP,kotlin
- Databases: MySQL, PostegreSQL, Mongo, Cassandra
- Tools: Atom, Aptana, Eclipse, Android Studio, Notepad++, Netbeans.
Having a degree in Computer Science enabled me to deeply learn most of the things regarding programming, and i believe that my understanding of problem solving and complex algorithms are also skills that have and will continue to contribute to my overall success as a developer.
I’ve worked on countless freelance projects and have been involved with a handful of notable startups. Also while freelancing I was involved in doing other IT tasks requiring the use of computers from working with data, content creation and transcription.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Calculate the value for the equilibrium constant for each of the following aqueous reactions. a. NH 3 + H 3 O + NH 4 + H 2 O b. NO 2 - + H 3 O + HNO 2 + H2O c. NH 4 + CH 3 CO 2 - NH3 + CH 3 CO 2 H...
-
a. Calculate the value of Ka for the following acids: i. 0.0200 mol dm 3 2-aminobenzoic acid, which has a pH of 4.30 ii. 0.0500 mol dm 3 propanoic acid, which has a pH of 3.10 iii. 0.100 mol dm 3...
-
A firm is engaged in the production of two types of products. The first product (A) earns a profit of $4 per unit and the second product (B) earns $7 per unit. The sales force requires that at least...
-
A trial balance was extracted from the books of V Baker, and it was found that the debit side exceeded the credit side by 40. This amount was entered in the suspense account. The following errors...
-
Richard Vanderbrooks home in New Orleans, Louisiana, was insured through Unitrin Preferred Insurance Co. His policy excluded coverage for, among other things, flood, surface water, waves, tidal...
-
Maltose, C12H22O11, is a sugar produced by malting (sprouting) grain. A solution of maltose at 25C has an osmotic pressure of 5.50 atm. What is the molar concentration of maltose?
-
Which supervisory skills would be most important to you in this situation and why?
-
Beaty Company has the following internal control procedures over cash receipts. Identify the internal control principle that is applicable to each procedure. (a) All over-the-counter receipts are...
-
Was having difficulty with this practice problem set. Could you please help? Will be sure to leave a review! Thank you so much! Assume you are given the following bonds: Bond A: a 2-year 3% annual...
-
Use the computer output for the radial keratotomy data of Problem 12 in Chapter 8, along with the additional output here, to answer the following questions. a. Perform the overall F test for the...
-
Consider the following four titrations (iiv): a. Rank the four titrations in order of increasing pH at the halfway point to equivalence (lowest to highest pH). b. Rank the four titrations in order of...
-
Consider a solution that contains both C 5 H 5 N and C 5 H 5 NHNO 3 . Calculate the ratio [C 5 H 5 N]/[C 5 H 5 NH + ] if the solution has the following pH values: a. pH = 4.50 b. pH = 5.00 c. pH =...
-
Which of the following statements is most true about the relative size of measures of variability? a. The standard deviation is usually larger than the variance. b. The variance is usually larger...
-
If the majority voting control partners in an entity are close to retirement, they may prefer more equity issued versus debt. T/F The more stable the selling price is, the more likely the firm ...
-
Explain the following concepts/topics. 1. Arbitration Tribunials under CUSMA as opposed to International Arbitration under the WTO or ICSID: 2. Lost or Not Lost clauses under a Marine Insurance...
-
Northfield Manufacturing has two operating divisions in a semiautonomous organizational structure. Americas Division, based in the United States, produces a specialized memory chip that is an input...
-
T 174 6. Cs has a half-life of 30.8 s and the number of nuclei present is 3.787 1016 nuclei. 55' Determine: a. Number of nuclei present 2.6 minutes later b. The activity of 17 CS at the time in a. c....
-
The parallel sides of the trapezoidal lot measure 160 m and 240 m are 40 m apart Find the distance of the dividing line from the 160m line parallel to the 2 sides that will divide the trapezoid into...
-
Should all public employees have a right to submit interest disputes to final and binding arbitration in exchange for giving up the right to engage in legal strike activity? Why or why not?
-
A genetically engineered strain of Escherichia coli (E. coli) is used to synthesize human insulin for people suffering from type I diabetes mellitus. In the following simplified reaction scheme,...
-
The acetoacetic ester synthesis cannot be used to make 3,3-dimethyl-2-hexanone. Explain why not.
-
The product of a Dieckmann cyclization can undergo alkylation, hydrolysis, and decarboxylation. This sequence represents an efficient method for preparing 2-substituted cyclopentanones and...
-
Identify the major product formed when each of the following compounds is treated with Et2CuLi followed by mild acid. (a) (b) (c) CN
-
Please solve As soon as i have only one hour Solve quickly i get you thumbs up directly Thank's Abdul-Rahim Taysir Faris, Karim and Issam are partners in a solidarity company, sharing profits and...
-
You are considering an investment in 20 year bonds issued by Moore Corporation. The bonds have no special covenants. The Wall Street Journal reports that 1-year T-bills are currently earning 6 30...
-
Tom incurred two casualties during 2020. The first involved his car, which was involved in an accident. Tom's basis in the car was $15,000. Prior to the accident, the car was worth $5,000; after the...

Study smarter with the SolutionInn App


