Classify each of the following reactions as a precipitation, acidbase, or gas-forming reaction. Show states for the
Question:
Classify each of the following reactions as a precipitation, acid–base, or gas-forming reaction. Show states for the products (s, ℓ, g, aq), and then balance the completed equation. Write the net ionic equation.
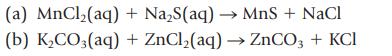
Transcribed Image Text:
(a) MnCl₂(aq) (b) K₂CO3(aq) + Na₂S(aq) → MnS + NaCl + ZnCl₂(aq) → ZnCO3 + KCI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
a MnCl2aq Na2Saq MnSs 2NaClaq This reaction is a precipitation reaction MnS is an insoluble solid pr...View the full answer

Answered By

Larlyu mosoti
I am a professional writer willing to do several tasks free from plagiarism, grammatical errors and submit them in time. I love to do academic writing and client satisfaction is my priority. I am skilled in writing formats APA, MLA, Chicago, and Harvard I am a statistics scientist and I can help out in analyzing your data. I am okay with SPSS, EVIEWS, MS excel, and STATA data analyzing tools.
Statistical techniques: I can do linear regression, time series analysis, logistic regression, and some basic statistical calculations like probability distributions. . I'm ready for your working projects!
Services I would offer:
• Academic writing.
• Article writing.
• Data entry.
• PDF conversion.
• Word conversion
• Proofreading.
• Rewriting.
• Data analyzing.
The best reason to hire me:
- Professional and Unique work in writing.
- 100% satisfaction Guaranteed
- within required time Express delivery
- My work is plagiarism Free
- Great communication
My passion is to write vibrantly with dedication. I am loyal and confident to give my support to every client. Because Client satisfaction is much more important to me than the payment amount. A healthy client-contractor relationship benefits in the longer term. Simply inbox me if you want clean work.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Classify each of the following reactions as a precipitation, acidbase, or gas-forming reaction. Show states for the products (s, , g, aq), and then balance the completed equation. Write the net ionic...
-
Classify each of the following reactions as one of the four possible types summarized in Table 19.3: (a) (b) (c) N2(g) 3 F2(g)2NF3(g) AH249 kJ; AS278 J/K N2(g) + 3C12(g) --> 2NC3(g) AH 460 kJ; AS...
-
The figure below represents a schematic of pipe network. A rate of 35 Ls, is pumped to feed two lines (3-4-5-6; and 2-7-8). The length and diameter of each pipe segment are listed in the table....
-
Between 1984 and 1985, the money supply in the United States increased to $641.0billion from $570.3billion, while that of Brazil increased to 106.1 billion cruzados from 24.4 billion. Over the same...
-
A space is filled up with a charge with volume density p p0 e-r3, where P0 and a are positive constants, r is the distance from the centre of this system. Find the magnitude of the electric field...
-
BASICS OF CAPITAL BUDGETING You recently went to work for Allied Components Company, a supplier of auto repair parts used in the after-market with products from Daimler AG, Ford, Toyota, and other...
-
The following transactions took place at Calhoun Counseling Services, a business established by Ronald Calhoun. INSTRUCTIONS For each transaction, set up T accounts from this list: Cash; Office...
-
Bay that the IN 1. e des not come benet round your intermediate contre und round the newers to the Date De De SE Apr 1. Transactions for Bend (Haldte Maturity Tovestment Rakya Mort Inc. is a general...
-
Balance each of the following equations, and classify them as precipitation, acidbase, gas-forming, or oxidationreduction reactions. Show states for reactants and products (s, , g, aq). (a) CuCl + HS...
-
Give a formula for each of the following compounds: (a) A soluble compound containing the bromide ion (b) An insoluble hydroxide (c) An insoluble carbonate (d) A soluble nitrate-containing compound...
-
Case studyspecific discussion questions and exercises are included at the end of each case study, so please review them for discussion and practice.
-
Rudy Gandolfi owns and operates Rudy's Furniture Emporium Incorporated. The balance sheet totals for assets, liabilities, and stockholders' equity at August 1, 2022, are as indicated. Described here...
-
The brand manager for a brand of toothpaste must plan a campaign designed to increase brand recognition. He wants to first determine the percentage of adults who have heard of the brand. How many...
-
Pulse rates of women are normally distributed with a mean of 77.5 beats per minute and a standard deviation of 11.6 beats per minute. Answer the following questions. What are the values of the mean...
-
Suppose youre applying a simulated annealing algorithm to a certain problem, where T is the parameter that measures the tendency to accept the current candidate to be the next trial solution. You...
-
Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together with comparative data for the year ended December 31, 2021. From the statement of cash flows...
-
On April 1, Adventures Travel Agency, Inc. began operations. The following transactions were completed during the month. 1. Issued common stock for $24,000 cash. 2. Obtained a bank loan for $7,000 by...
-
Which, if any, of the dichloroethene molecules drawn in Data Table II (3.) (4.) and (5.) are geometric isomers? A. B. C. D. cis-1,2-dichloroethene and trans-1,2-dichloroethene...
-
Benzoic acid, 1.35 g, is reacted with oxygen in a constant volume calorimeter to form H 2 O(l) and CO 2 (g) at 298 K. The mass of the water in the inner bath is 1.55 10 3 g. The temperature of the...
-
Calculate the P and T values for which Br2(g) is in a corresponding state to Xe(g) at 330. K and 72.0 bar.
-
For each of the following reactions, predict the product and draw the mechanism of its formation. a. b. c. d. e. f. 1) PhMgBr 2) H20 Me 1) NaCN 2) H20 *Me
-
Alpha Technology's work in process inventory on June 1 has a balance of $27,200 representing Job No. 72. During June, $68,900 of direct materials were requisitioned for Job No. 72 and $5,500 of...
-
Retained Earnings, Beginning Balance $600,000 Common Stock-no par: Beginning Balance 248,000 Net Income 89,000 Dividends Declared (23,000) Unrealized Gain on Available-for-Sale Investments-Net of tax...
-
Hill Industries had sales in 2019 of $7,520,000 and gross profit of $1,233,000. Management is considering two alternative budget plans to increase its gross profit in 2020. Plan A would increase the...

Study smarter with the SolutionInn App


