Like many metals, aluminum reacts with a halogen (here the orange-brown liquid Br 2 ) to give
Question:
Like many metals, aluminum reacts with a halogen (here the orange-brown liquid Br2) to give a metal halide, aluminum bromide. (The white solid on the lip of the beaker at the end of the reaction is Al2Br6.)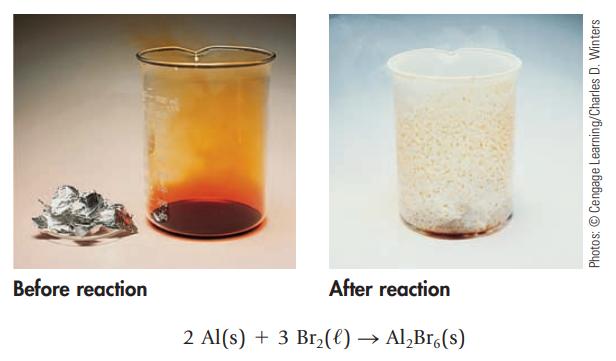
What mass of Br2, in grams, is required for complete reaction with 2.56 g of Al? What mass of white, solid Al2Br6 is expected?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





