The following photo shows copper balls, immersed in water, floating on top of mercury. What are the
Question:
The following photo shows copper balls, immersed in water, floating on top of mercury. What are the liquids and solids in this photo? Which substance is most dense? Which is least dense? 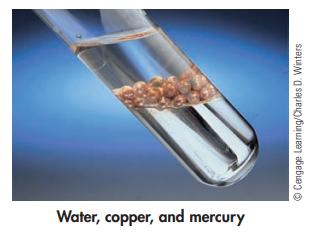
Transcribed Image Text:
Water, copper, and mercury ⒸCengage Learning/Charles D. Winters
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
The liquids in the photo are water and mercury The solids are the co...View the full answer

Answered By

FREDRICK MUSYOKI
Professional Qualities:
Solution-oriented.
Self-motivated.
Excellent problem-solving and critical thinking skills.
Good organization, time management and prioritization.
Efficient troubleshooting abilities.
Tutoring Qualities:
I appreciate students as individuals.
I am used to tailoring resources for individual needs.
I can integrate IT into student's lessons.
I am good at explaining concepts.
I am able to help students progress.
I have a wide curriculum knowledge.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The table below shows the melting points, boiling points and densities of substances A to D. a. Which substance is a gas at room temperature? b. Which substance is a liquid at room temperature? c....
-
Of all the substances (drugs and alcohol) discussed in the chapter, which substance is most closely connected to crime? Why do you think this is?
-
A solar pond consists of a thin layer of fresh water floating on top of a denser layer of salt water. When the salty layer absorbs sunlight it warms up and much of that heat is held there by the...
-
A 0.831-g sample of SO3 is placed in a 1.00-L container and heated to 1100 K. The SO3 decomposes to SO2 and O2: At equilibrium the total pressure in the container is 1.300 atm. Find the values of Kp...
-
A printing press priced at $315,000 is acquired by trading in a similar press and paying cash for the difference between the trade-in allowance and the price of the new press. a. Assuming that the...
-
The actuary for the pension plan of Flounder inc. calculated the following net gains and losses Incurred during the Year 2020 2021 2022 2023 (Gain) or Loss $302.100 479.800 (20.4000 1288.1000 Other...
-
3. Can you think of a work situation in which you experienced punishment? If so, did it benefit you (for example, by teaching you a valuable lesson)?
-
Growth Companys current share price is $20 and it is expected to pay a $1 dividend per share next year. After that, the firms dividends are expected to grow at a rate of 4% per year. a. What is an...
-
Use EXCEL ONLY - apply the Additive Seasonality approach for the following data set: PLEASE SHOW YOUR WORK. TABLE 2 COST OF LOCKERS [AS2] Year Per-Unit Labor Cost 1988 $80.15 1989 85.29 1990 85.75...
-
Categorize each of the following as an element, a compound, or a mixture. (a). Air (b). Fluorite (c). Brass (d). 18-carat gold
-
Identify the following as either physical changes or chemical changes. (a). The desalination of sea water (separation of pure water from dissolved salts). (b). The formation of SO 2 (an air...
-
The contribution of molecular vibrations to the molar internal energy Um of a gas of nonlinear N-atom molecules is (zero-point vibrational energy not included) Where us θs ¡ hvs/k...
-
E12-23 (Algo) (Supplement 12B) Preparing a Statement of Cash Flows, Indirect Method: T-Account Approach [LO 12-S2] Golf Goods Incorporated is a regional and online golf equipment retailer. The...
-
A symmetric compound channel in over bank flow has a main channel with a bottom width of 30 m, side slopes of 1:1, and a flow depth of 3m. The floodplains on either side of the main channel are both...
-
Solve these question in details and fully explaination. It is the pre-lab working for Capacitors. Thanks so much in advance. 1: The figure shows a circuit with a charged capacitor (left), two...
-
Exercise 10-14A (Algo) Straight-line amortization of a bond discount LO 10-4 Diaz Company issued bonds with a $112,000 face value on January 1, Year 1. The bonds had a 8 percent stated rate of...
-
1. What would we have to plot on the vertical axis? EXPLAIN YOUR ANSWER OR NO CREDIT. [Hint: Solve for k first.] F= Kx kx dala you Experi determine the K= K = F Cart SHOW ALL WORK OR NO CREDITI 2....
-
What is the client/server model?
-
An interest bearing promissory note for 90 days at 5.6% p.a. has a face value of $120,000. If the note is discounted 20 days after the issue date at a rate of 6.8% p.a., calculate the amount of...
-
Draw the mechanism of the following reaction: ONa + NaBr Br
-
Bromonium ions can be captured by nucleophiles other than water. Predict the products of each of the following reactions: a.
-
For each of the following objects determine whether or not it possesses a plane of symmetry: a. b. c. d. e. f.
-
03.16 Question Completion Status QUESTION 3 18 points Chapter 10. Bonds Payable On January 1, 2021,51,200,000, 5-year, bonds with a stated rate of 10% payable annually were issued for cash of...
-
The following is information for Palmer Company. \ table [ [ Cost of goods sold,Year 3 , Year 2 , Year 1 ] , [ Ending inventory,$ 6 4 3 , 8 2 5 , $ 4 2 6 , 6 5 0 , $ 3 9 1 , 3 0 0
-
Ingrid Tower is a 45 year old widow. Her Net Income For Tax Purposes consists of rental income. She maintains a home for herself and her two children. Her 19 year old son has a physical disability...

Study smarter with the SolutionInn App


