You have a mixture of oxalic acid, H 2 C 2 O 4 , and another solid
Question:
You have a mixture of oxalic acid, H2C2O4, and another solid that does not react with sodium hydroxide. If 29.58 mL of 0.550 M NaOH is required to titrate the oxalic acid in the 4.554-g sample to the second equivalence point, what is the mass percent of oxalic acid in the mixture? Oxalic acid and NaOH react according to the equation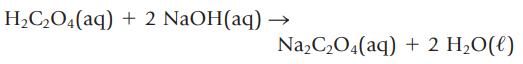
Transcribed Image Text:
H₂C₂O4(aq) + 2 NaOH(aq) Na₂C₂O4(aq) + 2 H₂O(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
To find the mass percent of oxalic acid in the mixture you can follow these steps Calculate the mole...View the full answer

Answered By

Munibah Munir
I've done MS specialization in finance’s have command on accounting and financial management. Forecasting and Financial Statement Analysis is basic field of my specialization. On many firms I have done real base projects in financial management field special forecasting. I have served more than 500 Clients for more than 800 business projects, and I have got a very high repute in providing highly professional and quality services.I have capability of performing extra-ordinarily well in limited time and at reasonable fee. My clients are guaranteed full satisfaction and I make things easy for them. I am capable of handling complex issues in the mentioned areas and never let my clients down.
4.60+
467+ Reviews
648+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
You have 0.954 g of an unknown acid, H 2 A, which reacts with NaOH according to the balanced equation If 36.04 mL of 0.509 M NaOH is required to titrate the acid to the second equivalence point, what...
-
A 2.500-g sample of a mixture of sodium carbonate and sodium chloride is dissolved in 25.00 mL of 0.798 M HCl. Some acid remains after the treatment of the sample. a. Write the net ionic equation for...
-
A 2.500-g sample of a mixture of sodium hydrogen carbonate and potassium chloride is dissolved in 25.00 mL of 0.437 M H2SO4. Some acid remains after treatment of the sample. a. Write both the net...
-
} S 1995 the # of Farms Century, data year 1935 1990 19.50 Aumcon people living on declined steadily during the shown by the Follow g as (in milion of persons) from 1935 19.55 1960 1965 11975 1980...
-
A manufacturer of printed circuit boards uses exponential smoothing with trend to forecast monthly demand of its product. At the end of December, the company wishes to forecast sales for January. The...
-
An aluminum alloy (2024) plate, heated to a uniform temperature of 227C, is al10wed to cool while vertically suspended in a room where the ambient air and surroundings are at 27C. The plate is 0.3 m...
-
Consider the following hypothesis test. The sample size is 120 and the population standard deviation is 5. Use .05. If the actual population mean is 9, the probability of a Type II error is .2912....
-
As the proprietor of Kingston Tires, Inc., you received the following invoice from a supplier: Requirements 1. Journalize the transaction required on September 23, 2012. 2. Journalize the return on...
-
Jason has a tax basis of $14,000 in his partnership interest at the beginning of the partnership tax year. The following amounts of partnership debt were allocated to Jason and are included in his...
-
(a) What is the pH of a 0.105 M HCl solution? (b) What is the hydronium ion concentration in a solution with a pH of 2.56? Is the solution acidic or basic? (c) A solution has a pH of 9.67. What is...
-
Sodium thiosulfate, Na 2 S 2 O 3 , is used as a fixer in black-and-white photography. Suppose you have a bottle of sodium thiosulfate and want to determine its purity. The thiosulfate ion can be...
-
Use inductive reasoning to predict the next three numbers in the pattern (or sequence). 1, 3, 4, 7, 11, 18, 29, 47, . . .
-
Pacifico Company, a U.S.-based importer of beer and wine, purchased 1,500 cases of Oktoberfest-style beer from a German supplier for 390,000 euros. Relevant U.S. dollar exchange rates for the euro...
-
Palmerstown Company established a subsidiary in a foreign country on January 1, Year 1, by investing 8,000,000 pounds when the exchange rate was $1.00/pound. Palmerstown negotiated a bank loan of...
-
Required information [The following information applies to the questions displayed below.] The following is financial information describing the six operating segments that make up Chucktown Sauce...
-
Question 1 (50 marks) Costa Ltd is a company with a 30 June year end. The following information relates to Costa Ltd and its subsidiary Jumbo for the year ended 30 June 20.22. Costa Ltd Jumbo Ltd Dr...
-
The following salaried employees of Mountain Stone Brewery in Fort Collins, Colorado, are paid semimonthly. Some employees have union dues or garnishments deducted from their pay. You do not need to...
-
After operating for several months, architect Paul Marciano completed the following transactions during the latter part of October: Journalize the transactions of Paul Marciano, Architect. Include an...
-
Prove that if Σ an is absolutely convergent, then a. an
-
Identify a systematic (IUPAC) name for each of the following compounds a. b. c. d. (e) CH 3 (CH 2 ) 4 CO 2 H (f) CH 3 (CH 2 ) 3 COCl (g) CH 3 (CH 2 ) 4 CONH 2 O: NH2
-
Identify the common name for each of the following compounds: a. b. c. d.
-
Draw the structures of eight different carboxylic acids with molecular formula C 6 H 12 O 2 . Then, provide a systematic name for each compound, and identify which three isomers exhibit chirality...
-
The ledger of Entity G at the end of the current year shows Accounts Receivable of $350,000. Bad debts are expected to be 5% of Accounts Receivable. Allowance for Doubtful Accounts has a credit...
-
Current Account and Statement of Financial Position Swan Monase trafont partie dealing in cosmetics and other assorted good. They share profit and desses in the ratio 3:2. The trial balance below was...
-
Your firm has the opportunity to repurpose one of its facilities and use it to invest in a new product that would change the profits of your firm for the next four years as follows: Year Before After...

Study smarter with the SolutionInn App


