In the previous problem, the reaction to produce XeF 2 occurs photochemically at room temperature. However, to
Question:
In the previous problem, the reaction to produce XeF2 occurs photochemically at room temperature. However, to produce XeF4(s), higher temperature and pressure are required. Production of XeF6 requires even higher pressures. Discuss the differences in reaction conditions for these three reactions in terms of activation energy and collision theory.
Previous problem
According to an article published in 1966 in the Journal of Chemical Education, xenon reacts photochemically with fluorine at room temperature (298 K) to produce XeF2. A portion of a table showing ratios of gases in the reaction vessel for four experiments is reproduced below. (Assume that atmospheric pressure is exactly 1 atm.) The reaction is as shown:
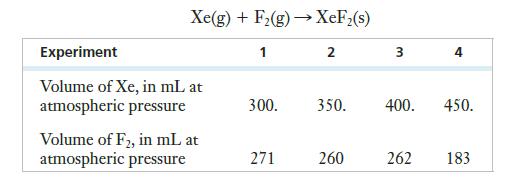
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





