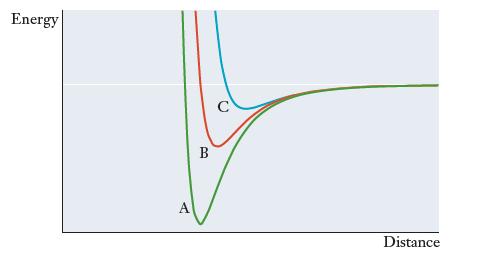
Nitrogen is capable of forming single, double, or triple bonds, and the figure that follows shows the
Question:
Nitrogen is capable of forming single, double, or triple bonds, and the figure that follows shows the potential energy as a function of internuclear distance for each of these types of bonds. Match the three curves in the figure (A, B, and C) to the three types of bonds. Explain your reasoning.
Transcribed Image Text:
Energy B C Distance
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Triple bonds are stronger and shorter than double bonds w...View the full answer

Answered By

Parvesh Kumar
I am an experienced Mathematics and Statistics tutor with 10 years of experience teaching students and working professionals. I love teaching students who are passionate to learn subjects or wants to understand any mathematics and statistics concept at graduation or master’s level. I have worked with thousands of students in my teaching career. I have helped students deal with difficult topics and subjects like Calculus, Algebra, Discrete Mathematics, Complex analysis, Graph theory, Hypothesis testing, Probability, Statistical Inference and more. After learning from me, students have found Mathematics and Statistics not dull but a fun subject. I can handle almost all curriculum of mathematics. I did B.Sc (mathematics), M.Sc (mathematics), M.Tech (IT) and am also Gate (CS) qualified. I have worked in various college and school and also provided online tutoring to American and Canadian students. I look forward to discussing with you and make learning a meaningful and purposeful
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The figure that follows shows ball-and-stick drawings of three possible shapes of an AF3 molecule. (a) For each shape, give the electron-domain geometry on which the molecular geometry is based. (b)...
-
A 230.0-g object on a spring oscillates left to right on a frictionless surface with a frequency of 2.00 Hz. Its position as a function of time is given by x = (8.00 cm) sin ( t.? (a) Sketch a graph...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
1Describe in a simplified manner the flow of information from gene to protein
-
Are there problems with scenario analysis? Define simulation analysis, and discuss its principal advantages and disadvantages. Shrieves Casting Company is considering adding a new line to its product...
-
Certain organizations regularly attack advertisers for their promotional methods. What could the advertising industry do to make themselves a smaller target for such criticisms? Be specific.
-
Is the contractor licensed and insured?
-
Bienestar Inc., has the following departmental structure for producing a well-known multivitamin: A consultant designed the following cellular manufacturing structure for the same product: The times...
-
A) The cash sales per a register tape were $591. The cash count is $558. B) The cash sales per a register tape were $9,300. The cash count is $8.820. Prepare the general journal entries to record the...
-
The N 5 + cation has been synthesized and studied. Consider the possible Lewis structure below. Indicate the hybridization expected for each nitrogen atom and the expected bond angles. Assuming that...
-
Nitrogen triiodide, NI 3 (s), is unstable and will spontaneously detonate to form a bright purple cloud of nitrogen and iodine gases accompanied with a loud bang, which suggests a release of energy....
-
Phuong Nguyen runs a commercial kitchen that produces a range of bottled Vietnamese cooking sauces that are sold in major retail food stores across the country. Past accounting records show that the...
-
21. How a degradation process is modeled? 22.Give the homogenity property in Linear Operator 23. Give the relation for degradation model for continuous function 24.which is called the superposition...
-
28. Define Gray-level interpolation 29. What is meant by Noise probability density function? 30. Why the restoration is called as unconstrained restoration? 31. Which is the most frequent method to...
-
34. Give the relation for guassian noise 35. Give the relation for rayleigh noise 36. Give the relation for Gamma noise 37. Give the relation for Exponential noise 38. Give the relation for Uniform...
-
41. What is pseudo inverse filter? 42. What is meant by least mean square filter? 43. Give the difference between Enhancement and Restoration PART-B 1. Discuss different mean filters
-
1.Discuss different mean filters 2. Draw the degradation model and explain. 3.Write short notes on Median Filters
-
Give specific examples in manufacturing for which artificial intelligence could be effective.
-
Assume you are the accountant for Catalina Industries. John Catalina, the owner of the company, is in a hurry to receive the financial statements for the year ended December 31, 20X1, and asks you...
-
Atomic emission experiments of a mixture show a calcium line at 422.673 nm corresponding to a 1 P 1 1 S 0 transition and a doublet due to potassium 2 P 3/2 2 S 1/2 and 2 P 1/2 2 S 1/2 transitions...
-
Consider the 1s np 3 P 1s nd 3 D transition in He. Draw an energy-level diagram, taking the spin-orbit coupling that splits terms into levels into account. Into how many levels does each term split?...
-
Calculate the transition dipole moment, for a transition from the 1s level to the 2p z level in H. Show that this transition is allowed. The integration is over r, θ, and . Use for the...
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid
-
uppose a taxpayer has exhausted his lifetime exclusion amount and has $14 million. a. Assuming a flat 40% gift tax rate, what is the maximum amount a taxpayer can transfer to her daughter (and still...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...

Study smarter with the SolutionInn App


