Suppose that speed distributions for each of the following gases were added to Figure 5.7. Which of
Question:
Suppose that speed distributions for each of the following gases were added to Figure 5.7. Which of the four gases in the original figure would each most closely resemble? Explain your answer.
(a) Ar
(b) Ne
(c) CH4
Figure 5.7
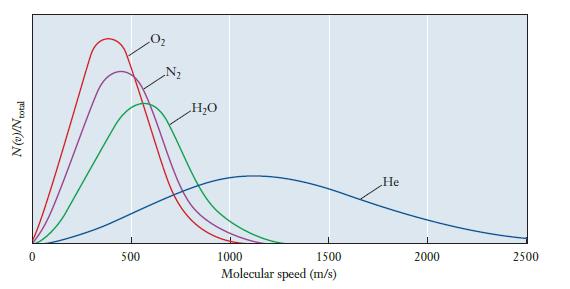
Transcribed Image Text:
N(v)/N total 0 500 N₂ H₂O 1000 1500 Molecular speed (m/s) He 2000 2500
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Because temperature is fixed the ...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
Explain the meaning of the terms emoluments, employments and office for the purposes of PAYE as you earn systems. 2. Explain the actual receipts basis of assessing the emoluments from the employment...
-
A 14-foot piece of string is cut into two pieces so that the longer piece is 2 feet longer than twice the shorter piece. Find the lengths of both pieces. What is the lenath of the shorter oiece?1...
-
Derby Company prepares monthly cash budgets. Relevant data from operating budgets for 2013 are: All sales are on account. Collections are expected to be 60% in the month of sale, 30% in the first...
-
Referring back to Exercise 6.4-19, find the likelihood ratio test of H0: = 1, unspecified, against all alternatives.
-
6. Repeat the previous problem, assuming that the dividend yield is 1.5%.
-
On January 1, 2010, Pine Grove Country Club purchased a new riding mower for $15,000.The mower is expected to have an 8-year life with a $1,000 salvage value . What journal entry would Pine Grove...
-
Bobby Brady has a full-time job as an electrical engineer for the oty ulty. In spare time, Bobby repairs electronic gear in the basement of his personal residence. Most of his business comes from...
-
Why do heavier gases move more slowly than light gases at the same temperature?
-
Use the kinetic theory to explain what happens to the pressure exerted by a gas as its temperature is increased.
-
Determine the range of values of P for which equilibrium of the block shown is maintained. , 3 0.25 - 0.20 500 N
-
On July 1, 2021, P Company borrowed P160,000 to purchase 80 percent of the outstanding common stock of S Company. This loan, carrying a 10 percent annual rate, is payable in 8 annual installments...
-
Case Analysis Strategic leaders, being at the highest level of an organization, are responsible for charting its path to success. They visualize an ideal picture of their enterprise in a futuristic...
-
3 Refrigerant-134a enters a adiabatic compressor at 100 kPa and -24C with a flow rate of 1.300 m/min and leaves at 800 kPa and 60C. Determine the mass flow rate of R-134a and the power input to the...
-
The following trial balance of Bramble Traveler Corporation does not balance. Bramble Traveler Corporation Trial Balance April 30, 2025 Debit Credit Cash $6,221 Accounts Receivable 5,350 Supplies...
-
From this analysis, we can see than the actual number of unit produced was actually less than the forecasted, yet the actual revenue gain from were greater than the forecasted one. This situation...
-
During 20X6, PepsiCo, Inc., had sales of $35.1 billion, operating profit of $6.4 billion, and net income of $5.6 billion. Earnings per share (EPS) were $3.42. On May 15, 20X7, of a share of PepsiCo's...
-
Evaluate how many lines there are in a true rotational spectrum of CO molecules whose natural vibration frequency is w = 4.09 1014 s1 and moment of inertia I = 1.44 1039 g cm2.
-
The amplitude of a pendulum consisting of a mass on a long wire is initially adjusted to have a very small value. The amplitude is found to decrease slowly with time. Is this process reversible?...
-
A process involving an ideal gas is carried out in which the temperature changes at constant volume. For a fixed value of T, the mass of the gas is doubled. The process is repeated with the same...
-
You are told that S = 0 for a process in which the system is coupled to its surroundings. Can you conclude that the process is reversible? Justify your answer.
-
7 . 4 3 Buy - side vs . sell - side analysts' earnings forecasts. Refer to the Financial Analysts Journal ( July / August 2 0 0 8 ) study of earnings forecasts of buy - side and sell - side analysts,...
-
Bond P is a premium bond with a coupon of 8.6 percent , a YTM of 7.35 percent, and 15 years to maturity. Bond D is a discount bond with a coupon of 8.6 percent, a YTM of 10.35 percent, and also 15...
-
QUESTION 2 (25 MARKS) The draft financial statements of Sirius Bhd, Vega Bhd, Rigel Bhd and Capella for the year ended 31 December 2018 are as follows: Statement of Profit or Loss for the year ended...

Study smarter with the SolutionInn App


