Question: We have seen the nuclear equation for the beta decay involved in radiocarbon dating. Now complete the equations for each of the following 2 decay
We have seen the nuclear equation for the beta decay involved in radiocarbon dating. Now complete the equations for each of the following 2 decay reactions, using 0 –1β to represent the beta particle:
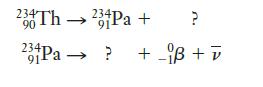
Strategy
We can use both the mass numbers and the charges given to determine the missing particles in the equations, much as in the previous example for alpha decay.
234Th 23+Pa+ 90 91 ? 23Pa ? +_i + v 91-
Step by Step Solution
3.40 Rating (153 Votes )
There are 3 Steps involved in it
Consider each equation separately starting with the thorium234 decay The fact that both Th and ... View full answer

Get step-by-step solutions from verified subject matter experts


