Consider the production of carbon dioxide and methane from carbon monoxide and hydrogen. Strategy Use Hesss law
Question:
Consider the production of carbon dioxide and methane from carbon monoxide and hydrogen.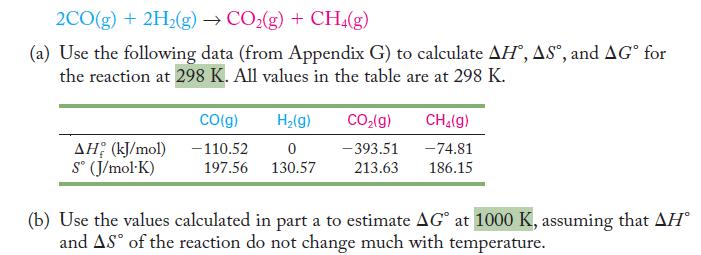
Strategy
Use Hess’s law to determine ΔH ° and ΔS °; then calculate ΔG °.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
At 1000 K the reaction is no longer spontaneous at standardstate conditions The result ...View the full answer

Answered By

Sufiyan Ahmed Tariq
I am a Chartered Accountant and an Associate Public & Finance Accountant. I also hold a bachelors of Commerce degree. I have over 8 years of experience in accounting, finance and auditing. Through out my career, I have worked with many leading multinational organisation.
I have helped a number of students in studies by teaching them key concepts of subjects like accounting, finance, corporate law and auditing. I help students understanding the complex situation by providing them daily life examples.
I can help you in the following subject / areas:
a) Accounting;
b) Finance;
c) Commerce;
d) Auditing; and
e) Corporate Law.
4.90+
7+ Reviews
17+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
The actual concentration of carbon dioxide in exhaled air is about 0.04, or 100 times the ambient concentration. Find the value of S that gives this as the equilibrium. The above problem investigates...
-
The kinetic data in the following table were obtained for the reaction of carbon dioxide and water to produce bicarbonate and hydrogen ion catalyzed by carbonic anhydrase: CO2 + H2O HCO-3 + H+ [H....
-
elow is selected financial information for SunRise Company. Selected Balance Sheet Data - As of Dec. 31, 2018 Dec. 31, 2017 Cash and short-term investments $ 958,245 $ 745,800 Accounts Receivable...
-
Treatment of an ?-amino acid with DCC yields a 2, 5-diketopiperazine. Propose a mechanism. H3N DCC H- N-H 4-N An a-amino acid A 2,5-diketopiperazine R.
-
Puff n Stuff Services is a small company that assembles mailings for clients in the Atlanta area. Different-size envelopes are stuffed with various items such as coupons, advertisements, political...
-
Is market research information used in making marketing decisions?
-
On January 1, 2010, the Stimpson Company sells land to Barker Company for $2.5 million, then immediately leases it back. The relevant information is as follows: 1. The land was carried on Stimpsons...
-
The number of countries that are expected to adopt IFRS as their home country standards by 2011 is: 200 167 150 117
-
Calculate the Gibbs free-energy change for the reaction of nitrogen monoxide and bromine to form nitrosyl bromide at 298 K under two sets of conditions. (a) The partial pressure of each gas is 1.0...
-
Calculate G and determine whether the following reaction will take place spontaneously under standard-state conditions at 298 K. Strategy Use the standard Gibbs free energies of formation from...
-
Refer to the data in PE 10-17. Assuming all interest has been accounted for, make the necessary journal entry (or entries) following transactions to record the retirement of the bonds at the end of...
-
Create your own privacy philosophy. This should cover the policies you will use for email, texting, social media, and internet usage. Consider what information is being collected anout you in each...
-
1. What future markets might be attractive to Carrefour and which mode of operation would be preferable? How important is the theoretical concept of psychological distance? 2. Corporate...
-
Briefly restate your problem space and methodology. Considering your problem space and methodology, what factors are you considering in deciding whether to use a theoretical foundation or a...
-
In light of your personal experience, what strategies or approaches do you believe could be effective in creating a workplace environment where employees from diverse cultural backgrounds feel both...
-
1. Entrepreneurs hold many common traits, identify five common traits of an entrepreneur that resonate with you and discuss each one of the five traits and why they matter to you. 2. Why is...
-
The following diagrams represent two electromagnetic waves. Which wave corresponds to the higher-energy radiation? Explain. a. b.
-
How can you tell from the vertex form y = a(x - h) 2 + k whether a quadratic function has no real zeros?
-
Compute points on the velocity profile from the pipe wall to the centerline of a 3/4-in Type K copper tube if the volume flow rate of water at 60F is 0.50 gal/min. Use increments of 0.05 in and...
-
Compute points on the velocity profile from the tube wall to the centerline of a plastic pipe, 125 mm OD 7.4 mm wall, if the volume flow rate of gasoline (sg = 0.68) at 25C is 3.0 L/min. Use...
-
A simple heat exchanger is made by welding one-half of a 1¾-in drawn steel tube to a flat plate as shown in Fig. 9.30. Water at 40°F flows in the enclosed space and cools the plate....
-
Deacon Company is a merchandising company that is preparing a budget for the three - month period ended June 3 0 th . The following information is available Deacon Company Balance Sheet March 3 1...
-
Mango Company applies overhead based on direct labor costs. For the current year, Mango Company estimated total overhead costs to be $460,000, and direct labor costs to be $230,000. Actual overhead...
-
Which of the following do we expect to be the horizon growth rate for a company (long term growth rate- say 30-50 years)? A) Inflation B) Industry Average C) Zero D) Market Beta

Study smarter with the SolutionInn App


