Give the hybrid orbital set used by each of the red atoms in the following molecules. :
Question:
Give the hybrid orbital set used by each of the red atoms in the following molecules.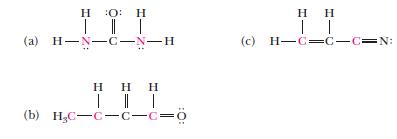
Transcribed Image Text:
: | || | (2) H-N-C-N-H (b) H,C=C=C=C=0 | | (c) H=C=C=C=N:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
To determine the hybrid orbitals used by atoms in molecules we have to look at the bonding and geome...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
What is the hybrid orbital set used by each of the underlined atoms in the following molecules? :: H-N-C-N-H (a) TIT (b) H,C=C=C=C=0 (c) H=C=C=C=N:
-
What hybrid orbital set is used by each of the indicated atoms in the molecules below? (a) The carbon atoms and the oxygen atom in dimethyl ether, CH 3 OCH 3 (b) Each carbon atom in propene (c) The...
-
What is the OSO angle and the hybrid orbital set used by sulfur in each of the following molecules or ions? (a) SO 2 (b) SO 3 (c) SO 3 2 (d) SO 4 2
-
Sound waves with frequency 3000 Hz and speed 343 m/s diffract through the rectangular opening of a speaker cabinet and into a large auditorium of length d = 100 m. The opening, which has a horizontal...
-
Sales Forecast Why do you think most long-term financial planning begins with sales forecasts? Put differently, why are future sales, the key input?
-
Indicate which of the following items would be reported as an extraordinary item in Mordica Corporations income statement. (a) Loss from damages caused by volcano eruption. (b) Loss from sale of...
-
Purchasing digital devices for children. PC Magazine (December 2019) reported the results of a survey of 1,000 parents of school-age children pertaining to the appropriate age the child should...
-
A crustal block with mean density 3000 kg m^3 is initially in is ostatic equilibrium with the surrounding rocks whose density is 3200 kg m^3, as in the figure (a). After subsequent erosion the...
-
Kardashian Company uses flexible budgets. At normal capacity of 8,000 units, budgeted manufacturing overhead is: $64,000 variable and $180,000 fixed. If Kardashian had actual overhead costs of...
-
Identify all the orbitals that form the bonds and hold the lone pairs on the central atom in the following molecules. (a) OF 2 (b) NH 3 (c) BCl 3
-
What orbitals on selenium and fluorine form the bonds in SeF 4 ? What orbital holds the lone pair on selenium?
-
If management chooses to reduce its selling price to match that of a competitor, how will the break-even point be affected?
-
Convex Productions produces full-length motion pictures for distribution worldwide. Convex has just purchased the rights to a movie script entitled Native Sun, which it intends to develop as its next...
-
You are visiting the Engineering Office of Denton Hospital, as part of a consulting project. You notice some charts on one wall which look familiar to you: One of the employees notices you reading...
-
Richmond Clinic has obtained the following estimates for its costs of debt and equity at different capital structures: What is the firms optimal capital structure? (Hint: Calculate its corporate cost...
-
Suppose a sample yields estimates \(\widehat{\theta}_{1}=5, \widehat{\theta}_{2}=3\), se \(\left[\widehat{\theta}_{1} ight]=2\), and se \(\left[\widehat{\theta}_{2} ight]=1\) and the correlation...
-
Helium expands in a nozzle from \(0.8 \mathrm{MPa}, 500 \mathrm{~K}\), and negligible velocity to \(0.1 \mathrm{MPa}\). Calculate the throat and exit areas for a mass flow rate of \(0.34 \mathrm{~kg}...
-
(a) When would you use split, splitless, or on-column injection in gas chromatography? (b) Explain how solvent trapping and cold trapping work in splitless injection.
-
Determine the reactions in supports A and D and connections B and C. Sketch its shear and moment diagram and determine the magnitude ankoration of the maximum shear and moment for every member. 18 3...
-
The hollow shaft has the cross section shown and is made of an elastic perfectly plastic material having a yield shear stress of t Y . Determine the ratio of the plastic torque T p to the maximum...
-
A circular shaft having a diameter of 4 in. is subjected to a torque of 250 kip # in. If the material is elastic perfectly plastic, with Y = 16 ksi, determine the radius of the elastic core.
-
The shaft is subjected to a maximum shear strain of 0.0048 rad. Determine the torque applied to the shaft. 2 in.- 7 (ksi) 12 Y (rad) 0.0006 0.0048
-
Hrubec Products, Incorporated, operates a Pulp Division that manufactures wood pulp for use in the production of various paper goods. Revenue and costs associated with a ton of pulp follow: Selling...
-
The AICPA guidelines suggest that taxes should be transparent and visible. This means that: a. The taxes affect similarly situated taxpayers in a similar manner. b. Taxes should be due at the same...
-
What is Apple Companys strategy for success in the marketplace? Does the company rely primarily on customer intimacy, operational excellence, or product leadership? What evidence supports your...

Study smarter with the SolutionInn App


