Histidine is an essential amino acid that the body uses to form proteins. The Lewis structure of
Question:
Histidine is an essential amino acid that the body uses to form proteins. The Lewis structure of histidine follows. What are the approximate values for bond angles 1 through 5 (indicated on the structure by blue numbers)?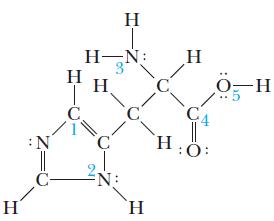
Transcribed Image Text:
H :N C H-N: H H C. H C N: C. H H C4 :O: -H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
angle 1 120 ...View the full answer

Answered By

Shem Ongek
I am a professional who has the highest levels of self-motivation. Additionally, I am always angled at ensuring that my clients get the best of the quality work possible within the deadline. Additionally, I write high quality business papers, generate quality feedback with more focus being on the accounting analysis. I additionally have helped various students here in the past with their research papers which made them move from the C grade to an A-grade. You can trust me 100% with your work and for sure I will handle your papers as if it were my assignment. That is the kind of professionalism that I swore to operate within. I think when rating the quality of my work, 98% of the students I work for always come back with more work which therefore makes me to be just the right person to handle your paper.
4.80+
174+ Reviews
426+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Various amino acids have utility as food additives and in medical applications. They are often synthesized by fermentation using a specific microorganism to convert a substrate (e.g., a sugar) into...
-
The four intermediates of the urea cycle are -amino acids. (a) Which one of these is considered to be an essential amino acid in children? (b) Outline a pathway by which adults can synthesize this...
-
The study of carbon- containing compounds and their properties is called organic chemistry. Besides carbon atoms, organic compounds also can contain hydrogen, oxygen, and nitrogen atoms (as well as...
-
In a recent year, the total scores for a certain standardized test were normally distributed, with a mean of 500 and a standard deviation of 10.4. Answer parts (a)-(d) below. (a) Find the probability...
-
Could a companys change in NWC be negative in a given year? Explain how this might come about. What about net capital spending?
-
Assume that you are applying for a part-time job as an accounting clerk in a retail clothing establishment. During the interview, the store manager asks how you expect to contribute to the business....
-
In baseball, a "no-hitter" is a regulation 9-inning game in which the pitcher yields no hits to the opposing batters. Chance (Summer 1994) reported on a study of nohitters in Major League Baseball...
-
The Artisan Wines is a retail store selling vintage wines. On December 31, 2019, the firms general ledger contained the accounts and balances below. All account balances are normal. Cash ..$ 28,386...
-
Mojo Mining has a bond outstanding that sells for $2,102 and matures in 20 years. The bond pays semiannual coupons and has a coupon rate of 6.5 percent. The par value is $2,000. If the company's tax...
-
Draw the molecular orbital diagrams for NO - and NO + . Compare the bond orders in these two ions.
-
Phosgene, COCl 2 , is a highly toxic gas that was used in combat during World WarI . $3It is an important intermediate in the preparation of a number of organic compounds but must be handled with...
-
The water in a tank is pressurized by air, and the pressure is measured by a multifluid manometer as shown in Fig. P312. Determine the gage pressure of air in the tank if h 1 = 0.4 m, h 2 = 0.6 m,...
-
A jury of 12 is to be created from a pool of 20 men and 10 women. What is the probability that all 12 on the jury will be men?
-
Reproduced below from Farthington Supply's accounting records is the accounts receivable subledger along with selected general ledger accounts. Dec. 31/19 Balance Credit sales in 2020 General Ledger...
-
Linear Correlation Coefficient In Exercises 9-12, the linear correlation coefficient r is provided. Use Table 2-11 to find the critical values of r. Based on a comparison of the linear correlation...
-
Problem 5-4A Adjusting entries and multi-step income statement-perpetual LO5 Use the unadjusted trial balance of Electric Bike on December 31, 2020. Cash Accounts receivable Merchandise inventory...
-
Only Brakes Inc. is a start-up company that raised the following debt capital in its first year: notes payable of $10,000,000; long-term bank debt of $35,000,000; and bonds payable of $60,000,000....
-
An electrospray/transmission quadrupole mass spectrum of the -chain of hemoglobin from acidic solution exhibits nine peaks corresponding to M n+ n . Find the charge, n, for peaks A-I. Calculate the...
-
Hotel Majestic is interested in estimating fixed and variable costs so that the company can make more accurate projections of costs and profit. The hotel is in a resort area that is particularly busy...
-
The box beam is made of an elastic perfectly plastic material for which Ï Y = 250 MPa. Determine the residual stress in the top and bottom of the beam after the plastic moment M p is applied and...
-
Determine the shape factor of the cross section. fototod
-
Determine the shape factor for the member having the tubular cross section. 2d
-
September 1 . Purchased a new truck for $ 8 3 , 0 0 0 , paying cash. September 4 . Sold the truck purchased January 9 , Year 2 , for $ 5 3 , 6 0 0 . ( Record depreciation to date for Year 3 for the...
-
Find the NPV for the following project if the firm's WACC is 8%. Make sure to include the negative in your answer if you calculate a negative. it DOES matter for NPV answers
-
What is the value of a 10-year, $1,000 par value bond with a 12% annual coupon if its required return is 11%?

Study smarter with the SolutionInn App


