In each part, arrange the orbitals in order of increasing energy in a multielectron atom. 4s (a)
Question:
In each part, arrange the orbitals in order of increasing energy in a multielectron atom.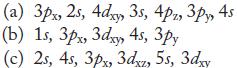
Transcribed Image Text:
4s (a) 3px, 2s, Adxy, 35, 4, 3, 45 (b) 1s, 3px, 3dxy 4s, зру (c) 2s, 4s, 3px, 3dxz, 5s, 3xy
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Heres the order of increasing energy for the orbitals in each pa...View the full answer

Answered By

Shadrack Mulunga
I am a Biochemistry by profession. However, I have explored different fields of study. My quest to explore new fields has helped me gain new knowledge and skills in Business, clinical psychology, sociology, organizational behavior and general management, and Project Management. I count my expertise in Project management, in particular, creation of Work Break Down Structure (WBS) and use of Microsoft Project software as one of my greatest achievement in Freelancing industry. I have helped thousands of BSC and MSC students to complete their projects on time and cost-effectively using the MS Project tool. Generally, I find happiness in translating my knowledge and expertise to success of my clients. So far, i have helped thousands of students to not only complete their projects in time but also receive high grades in their respective courses. Quality and timely delivery are the two key aspects that define my work. All those who hired my services always come back for my service. If you hire my services today, you will surely return for more. Try me today!
5.00+
154+ Reviews
289+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
In each part, arrange the subshells in order of increasing energy in a multielectron atom. (a) 5p, 2p, 3d, 25, 3 (b) 1s, 2p, 3d, 2s, 4d, 3s (c) 1s, 2s, 3s, 2p, 3p, 4p, 3d
-
List all orbitals from 1s through 5s according to increasing energy for multielectron atoms.
-
In order to develop interesting new compounds inorganic chemists often start by noting the location of an element in the periodic table and its valence electron configuration. Suppose you are working...
-
A bank reconciliation takes time and must balance. An employee was struggling in balancing the bank reconciliation. Her supervisor told her to plug (make an unsupported entry for) the difference,...
-
Julie has been hired to help paint the trim of a building, but she is not convinced of the safety of the apparatus. A 5.0-m plank is suspended horizontally from the top of the building by ropes...
-
A particle of charge q > 0 is moving at speed v in the +z-direction through a region of uniform magnetic field B. The magnetic force on the particle is F = F0 (3i + 4j), where F0 is a positive...
-
There is variation in peoples attitudes toward how big the governments role should be. Does race and ethnicity play a role in explaining this variation? Run a regression equation using the ANES...
-
Luthan Company uses a predetermined overhead rate of $23.40 per direct labor-hour. This predetermined rate was based on 11,000 estimated direct labor-hours and $257,400 of estimated total...
-
9. Opportunity Cost is best defined as (1 Point) The amount of debt you take on by making a decision The price you pay to purchase something The benefit you gain by making a decision the value of the...
-
Unicom is a regulated utility serving Northern Illinois. The following table lists the stock prices and dividends on Unicom from 1989 to 1998. a. Estimate the average annual return you would have...
-
The absorption spectra of ions have been used to identify the presence of the elements in the atmospheres of the Sun and other stars. (In fact, the element helium was discovered in the spectrum of...
-
For all elements with Z 10, write the electron configuration for (a) Those that have two unpaired electrons. (b) The element with the largest number of unpaired electrons. (c) Those that have only...
-
What five factors are compensation policies usually based on? Name three employee benefits that are required by law. Name three employee benefits that firms often provide voluntarily.
-
10.) Steam enters a well-insulated turbine at 6 MPa, 400C and expands to 200 kPa, saturated vapor at a rate of 10 kg/s. (a) Draw a schematic of the process (5 pts). (b) Determine the exergy...
-
4. [8 marks] The tides in the Bay of Fundy are some of the largest in the world. The height, h(t), of the tide in meters after t hourse can be modeled by 39 h(t) = 25 con (77) + 30 4 COS 6 (a) What...
-
Wolfe, Inc. had credit sales for the period of $144,000. The balance in Allowance for Doubtful Accounts is a debit of $653. If Wolfe estimates that 2% of credit sales will be uncollectible, what is...
-
Water at 20C is to be pumped from a reservoir (ZA = 5 m) to another reservoir at a higher elevation (ZB = 13 m) through two 36-m- long pipes connected in parallel as shown. The pipes are made of...
-
Delph Company uses a job-order costing system with a plantwide predetermined overhead rate based on machine-hours. At the beginning of the year, the company estimated that 53,000 machine-hours would...
-
Aunt Maude frequently complains of a "draft" while sitting next to a window in her New York apartment in the winter, and she also says her feet get cold. She remarks that the window seems to leak...
-
Nate prepares slides for his microscope. In 1 day he prepared 12 different slides. Which equation best represents y, the total number of slides Nate prepares in x days if he continues at this rate? A...
-
What does a partition function represent? Can you describe this term using concepts from probability theory?
-
Explain the significance of the Boltzmann distribution. What does this distribution describe?
-
Why is the probability of observing a configuration of energy different from the Boltzmann distribution vanishingly small?
-
Aecerty 1067687 was completed with the folowing charaderistick Murulectere sec00 5xs:99 s35ida sputed
-
Assume todays settlement price on a CME EUR futures contract is $1.3180 per euro. You have a long position in one contract. EUR125,000 is the contract size of one EUR contract. Your performance bond...
-
Q2. Company ABC bought an equipment for $20,000 in 2015, with useful life of 5 years $5,000 residual value amortized using straight-line method. Prepare a table to illustrate the differences...

Study smarter with the SolutionInn App


