The standard Gibbs free-energy change for the following reaction is +55.69 kJ . Strategy Because we know
Question:
The standard Gibbs free-energy change for the following reaction is +55.69 kJ .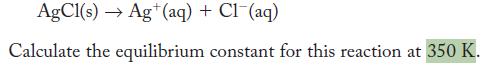
Strategy
Because we know the ΔG ° and the temperature, we can use Equation 17.11 to determine the equilibrium constant.
Equation 17.11![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
We should convert the AG to units of J AG ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Reactions between gases in the atmosphere are not at equilibrium, but for a thorough understanding of them we need to study both the rates at which they take place and their behavior under...
-
Calculate the standard Gibbs free energy change of reaction in each of the following using the standard molar values for Gibbs free energy change given here. In each case, comment on whether the...
-
Hydrogen iodide, HI, is used as a reagent in organic chemistry to transform primary alcohols into alkyl iodides. Suppose you are a chemist using HI; you would need to understand the equilibrium...
-
Recruitment and selection involves the following except: a) building a pool of candidates. b) applicants completing application forms. c) downsizing the organization. d) employment planning and...
-
Phenyl 4-aminosalicylate is a drug used in the treatment of tuberculosis. Propose a synthesis of this compound starting from 4-nitrosalicylicacid. CO2H H2N O2N Phenyl 4-aminosalicylate...
-
Jericho Vehicles manufactures special-purpose all-terrain vehicles primarily for the military and government agencies in the United States and for foreign governments. The company is planning to bid...
-
Does the organization position itself well against its competitors?
-
At the end of 2010, its first year of operations, the Swelland Company reported a pretax operating loss of $32,000 for both financial reporting and income tax purposes. At that time the company had...
-
A project has a forecasted cash flow of $117 in year 1 and $128 in year 2. The interest rate is 8%, the estimated risk premium on the market is 11.75%, and the project has a beta of 0.57. If you use...
-
The vapor pressure of water at 25 C is 23.77 torr , increasing to 42.20 torr at 35 C . Calculate the standard free energy and standard enthalpy changes at 25 C for the vaporization of water. Strategy...
-
The equilibrium constant for the following reaction is 1.0 10 14 at 298 K . Strategy We know that G = -RT ln K eq , and we have values for K eq and T. Use the proper value and units for R so the...
-
Michael P. Babine was injured when he was thrown from an El Toro mechanical bull that he rode at a nightclub. The club had placed mattresses around the bull to cushion the fall of riders, but the...
-
What work trait differences are similar in chart 1 and chart 2? Provide a comment for each of the 4 generations from each chart. Which work trait differences vary from those identified in chart 1 and...
-
Given the ALU design illustrated below, without changing the circuit design, please use the ALU to perform a logic NAND operation. Find out what the control signals should be (i.e. the values of...
-
Problem #5: Using the method of joints, determine the force in each member. State whether each member is in compression or tension. If the largest force each member can support is 4kN tension and 3kN...
-
Your cultural/social background and that of your family. What language, policies/structures and customs are relevant to your own culture? How do you think your own background impacts on people from...
-
In this second Case Assignment, the assignment is going to test your understanding of how successful teams operate efficiently through teamwork. Teamwork relies upon individuals to work together to...
-
Sodium metal requires a photon with a minimum energy of 4.41 10-19J to emit electrons. (a) What is the minimum frequency of light necessary to emit electrons from sodium via the photoelectric...
-
(a) Water flows through the nozzle of a garden hose. Find an expression for m in terms of line pressure P 1 , ambient pressure P 2 , inside hose diameter D 1 , and nozzle outlet diameter D 2 . Assume...
-
Convert 0.008 ft 3 /s to gal/min.
-
An existing fixture inserts the velocity probe described in Problem 9.5 exactly 60.0 mm from the outside surface of the pipe. If the probe reads 2.48 m/s, compute the actual average velocity of flow,...
-
An alternative scheme for using the velocity probe described in Problem 9.5 is to place it in the middle of the pipe, where the velocity is expected to be 2.0 times the average velocity. Compute the...
-
Your company BMG Inc. has to liquidate some equipment that is being replaced. The originally cost of the equipment is $120,000. The firm has deprecated 65% of the original cost. The salvage value of...
-
1. What are the steps that the company has to do in time of merger transaction? And What are the obstacle that may lead to merger failure? 2.What are the Exceptions to not to consolidate the...
-
Problem 12-22 Net Present Value Analysis [LO12-2] The Sweetwater Candy Company would like to buy a new machine that would automatically "dip" chocolates. The dipping operation currently is done...

Study smarter with the SolutionInn App


