A saturated solution of Cd(OH) 2 is shown in the middle beaker. If hydrochloric acid solution is
Question:
A saturated solution of Cd(OH)2 is shown in the middle beaker. If hydrochloric acid solution is added, the solubility of Cd(OH)2 will increase, causing additional solid to
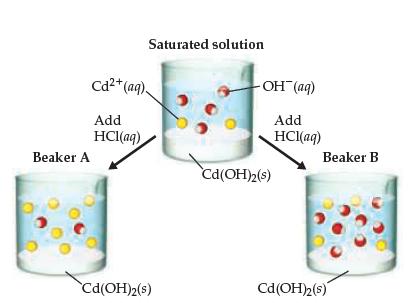
dissolve. Which of the two choices, Beaker A or Beaker B, accurately represents the solution after equilibrium is reestablished? (The water molecules and CI- ions are omitted for clarity.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus
Question Posted:





