Question: Based on the molar conductance values listed here for the series of platinum(IV) complexes, write the formula for each complex so as to show which
Based on the molar conductance values listed here for the series of platinum(IV) complexes, write the formula for each complex so as to show which ligands are in the coordination sphere of the metal. By way of example, the molar conductances of 0.050 M NaCl and BaCl2 are 107 ohm-1 and 197 ohm-1, respectively.
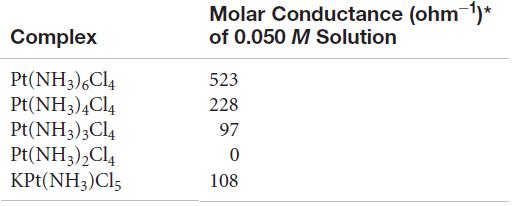
Molar Conductance (ohm 1)* Complex of 0.050 M Solution Pt(NH3),Cl4 Pt(NH3),Cl4 Pt(NH3)3Cl4 Pt(NH3),Cl4 KPt(NH3)Cl5 523 228 97 108
Step by Step Solution
3.38 Rating (157 Votes )
There are 3 Steps involved in it
The molar conductance values of the series of platinumIV complexes suggest that the number of ions p... View full answer

Get step-by-step solutions from verified subject matter experts


