Given the following bond-dissociation energies, calculate the average bond enthalpy for the Ti-Cl bond. AH(kJ/mol) TiCl4(g) TiCl;(g)
Question:
Given the following bond-dissociation energies, calculate the average bond enthalpy for the Ti-Cl bond.
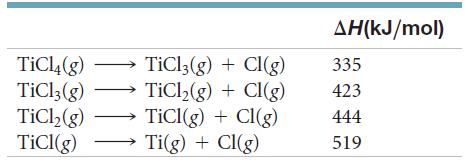
Transcribed Image Text:
AH(kJ/mol) TiCl4(g) TiCl;(g) TİC,(g) TICI(g) TiCl3(g) + Cl(g) TİCI,(g) + CI(g) TiCI(g) + CI(g) Ti(g) + CI(g) 335 423 444 519
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
Average bond energy of TiC1 bond TiC1 4 g TiC1 3 ...View the full answer

Answered By

Umair Expert
Hi Everyone.
I have 6 years of teaching experience.
I am serving as a tutor 2 more websites.
I will provide you projects and solutions of questions related to any subject.
I am a good programmer.(PHP, Python)
I am good command in mathematics.
I am here to assist you.
I'll be very happy to work with you...
Thanks dear..
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 978-0321696724
12th edition
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
Question Posted:
Students also viewed these Sciences questions
-
Basing your answers on the bond dissociation energies in Table 4.3, calculate which of the following reactions are endothermic and which are exothermic: (a) (CH3)2CHOH + HF (CH3)2CHF + H2O (b)...
-
a. Propose a mechanism for the following reaction: b. Given that the Ho value for the reaction is -42 kcal/mol and the bond dissociation energies for the C--H, C--Cl and O--H bonds are 101, 82, and...
-
Basing your answers on the bond dissociation energies in Table 4.3, calculate which of the following reactions are endothermic and which are exothermic: (a) (CH3)2CHOH + HF - (CH3)2CHF + H2O (b)...
-
What other types of contingency planning should Matt and Chris include to make the report comprehensive? Please explain the relevance of each suggestion.
-
If the double-stem display still has too few stems, we may wish to construct a stem and-leaf display with a separate stem to hold leaves 0 and 1, 2 and 3, 4 and 5, 6 and 7, and a stem to hold 8 and...
-
Describe the advantages and disadvantages associated with just-in-time inventory control. LO2
-
What are the uses, strengths, and weaknesses of the following approaches to organizational effectiveness? LO.1
-
Alex Franks was a guest staying at a Comfort Inn in Searcy, Arkansas, while he was working on a highway project. Franks found a bundle of money in plain view in the left part of the left drawer in...
-
A machine costing Php700,000 has a life of 10 years. The total depreciation at the end of 4 years is Php254,000 computed using straight line method. What is the salvage value of the machine? a)...
-
In May of the current year, your employer received a PIER report from the CRA that identified Canada Pension Plan (CPP) contribution deficiencies for employees in the organization who: turned 18...
-
Which of the following bonds are polar: (a) B - F (b) Cl Cl (c) Se O (d) H I Which is the more electronegative atom in each polar bond?
-
(a) If these three balloons are all the same size, what angle is formed between the red one and the green one? (b) If additional air is added to the blue balloon so that it gets larger, what happens...
-
Indicate the metric unit of measurement that would best express the following. The temperature of the water in an ocean.
-
The adjusted trial balance section of Menlo Company's worksheet shows a \(\$ 1,500\) debit balance in utility expense. At the end of the accounting period the accounting manager accrues an additional...
-
Identify each of the 10 amount columns of the worksheet and indicate to which column the adjusted balance of the following accounts would be extended: a. Accounts Receivable b. Accumulated...
-
Using the data from Table 3.3, show the effect on world output if each country moved toward specialization in the production of its comparative-disadvantage good. TABLE 3.3 Comparative Advantage as a...
-
The Professional Winner was RJ Andrews from Info We Trust, for the video Are Gazelles Endangered? (a) Watch this video. What data are this video conveying? (b) You can interact with the data and...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located outside the focal length of a converging lens. (b) Is the image real or virtual? (c) Is it upright or...
-
The system of Prob. 5/101 is repeated here. Crank OA is rotating at a counterclockwise angular rate of 9 rad /s, and this rate is decreasing at 5 rad/s 2 . Determine the angular acceleration a AB of...
-
Define cultural intelligence. Cite the books or journal articles you found in Capella's library. Explain why cultural intelligence is important for HR practitioners and other organizational managers.
-
Consider subsonic flow in a converging nozzle with fixed inlet conditions. What is the effect of dropping the back pressure to the critical pressure on? (a) The exit velocity, (b) The exit pressure,...
-
Consider gas flow through a converging nozzle with specified inlet conditions. We know that the highest velocity the fluid can have at the nozzle exit is the sonic velocity, at which point the mass...
-
How does the parameter Ma* differ from the Mach number Ma?
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


