One of the molecular orbitals of the H 2 - ion is sketched below: (a) Is the
Question:
One of the molecular orbitals of the H2- ion is sketched below:
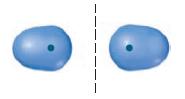
(a) Is the molecular orbital a σ or π MO? Is it bonding or antibonding?
(b) In H2-, how many electrons occupy the MO shown above?
(c) What is the bond order in the H2- ion?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
a In the diagram provided we see that there is orbital are aligned l...View the full answer

Answered By

Pankti Doshi
I have completed by Bachelors degree in Commerce in 2014 from Mumbai university and is a CFA student who has completed Level 1 in June-19.My More than 4 years of experience in Finance has refined my knowledge and expertise in accountancy and World finance.My tutoring experience began with my passion for teaching since I was in High school.I understand my student needs and try to take my teaching to a level to meet every students intellectual capacity to serve them better.I am really energetic,passionate and a great communicator.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:
Students also viewed these Sciences questions
-
The iodine bromide molecule, IBr, is an inter-halogen compound. Assume that the molecular orbitals of IBr are analogous to the homo nuclear diatomic molecule F2. (a) Which valence atomic orbitals of...
-
If one were to try to draw the simplest Lewis structure for molecular oxygen, the result might be the following However, it is known from the properties of molecular oxygen and experiments that O2...
-
In the ground state of mercury (Hg), a. How many electrons occupy atomic orbitals with n = 3? b. How many electrons occupy d atomic orbitals? c. How many electrons occupy pz atomic orbitals? d. How...
-
At the beginning of the current season, the ledger of Highland Tennis Shop showed Cash $2,500; Inventory $1,700; and Common Stock $4,200. The following transactions were completed during April. Apr....
-
Given that f(x) = x - 7 / x + 5, find each of the following. (a) f(7) (b) f(x + 1) (c) f(-5) (d) f (- 1/2)
-
Describe Assess debt features and their implications
-
Annual US craft beer production. Refer to Exercise 14.7 (p. 14-10) and the data on US beer production (in millions of barrels) for the years 20042019. a. Use the 20042017 values to forecast the 2018...
-
Mary Givens and Peggy Moser are partners engaged in operating The G&M Doll Shop, which has employed the following persons since the beginning of the year: T. Binn (general office...
-
28. An account was opened with $1,000 ten years ago. Today, the account balance is $1,500. If the account paid interest compounded annually, how much interest on interest was earned? A) $86.20 B)...
-
Suppose (X, Y) are distributed uniformly on the circle of radius 1 centered at the origin. Find the marginal densities of X and Y. Are X and Y independent?
-
The vapor pressure of ethanol (C 2 H 5 OH) at 19C is 40.0 torr. A 1.00-g sample of ethanol is placed in a 2.00 L container at 19C. If the container is closed and the ethanol is allowed to reach...
-
Rank the following gases from least dense to most dense at 1.00 atm and 298 K: SO 2 , HBr, CO 2 . Explain.
-
Using the amounts below, calculate the inventory turnover ratio , average days in inventory, and gross profit ratio. Net sales......................................$200,000 Cost of goods...
-
A year-end cut-off error occurred in 2017. A large shipment of nonperishable supplies arrived from South America on the last day of 2017 and had been left in the shipping containers outside the main...
-
15. [5] It's not so difficult to incorporate time-varying volatility into the BSM model as long as the time variation is not random. Assume a BSM economy, but this time, assume that the volatility of...
-
3.6. Explain and discuss the potential benefits to be gained by using blade twist, plan- form taper, low solidity, large radius, and low rotational speed for the main rotor of a heavy lift helicopter...
-
2. A VRM (Voltage Regulator Modul) is used to supply the voltageto the CPU of a computer. In the new generation of microprocessors,whose power consumption is 100W, the input voltage to the VRM is12V...
-
Alvarado Company produced 6,400 units of product that required 5.5 standard direct labor hours per unit. The standard variable overhead cost per unit is $5.80 per direct labor hour. The actual...
-
Building upon PP 13.1, create an array implementation of a map. PP 13.1 Create an array based implementation of a set called ArraySet that implements the Set interface.
-
r = 0.18 Find the coefficients of determination and non-determination and explain the meaning of each.
-
You need to know the melting point for CaC1 2 , for a lab report you are writing. Your lab partner says that the Handbook of Chemistry and Physics lists this as 68 o C. Do you think you should trust...
-
Show a Lewis structure for A1C1 4 . What are the formal charges on the atoms of this anion? What is its shape?
-
Ammonium cyanate is composed of an ammonium caution (NH 4 + ) and a cyanate anion (OCN ). Show a Lewis structure for the cyanate anion. (Both O and N are bonded to C.) Which atom has the negative...
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


