The color and wavelength of the absorption maximum for [Ni(H 2 O) 6 ] 2+ , [Ni(NH
Question:
The color and wavelength of the absorption maximum for [Ni(H2O)6]2+, [Ni(NH3)6]2+, and [Ni(en)3]2+ are given in Figure 23.30. The absorption maximum for the [Ni(bipy)3]2+ ion occurs at about 520 nm.
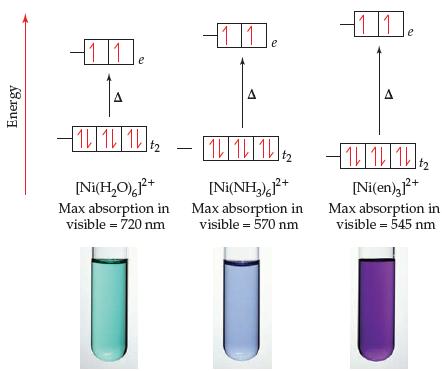
(a) What color would you expect for the [Ni(bipy)3]2+ ion?
(b) Based on these data, where would you put bipy in the spectrochemical series?
Transcribed Image Text:
11 11 11 A IL 1L 12 1 1 1 [Ni(H,O0),F+ Max absorption in visible = 720 nm [Ni(en),+ [Ni(NH,),P* Max absorption in visible = 570 nm Max absorption in visible = 545 nm Energy
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (15 reviews)
a Green and Violet It absorbs 725 nm red light and appears as the complem...View the full answer

Answered By

Madhur Jain
I have 6 years of rich teaching experience in subjects like Mathematics, Accounting, and Entrance Exams preparation. With my experience, I am able to quickly adapt to the student's level of understanding and make the best use of his time.
I focus on teaching concepts along with the applications and what separates me is the connection I create with my students. I am well qualified for working on complex problems and reaching out to the solutions in minimal time. I was also awarded 'The Best Tutor Award' for 2 consecutive years in my previous job.
Hoping to get to work on some really interesting problems here.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:
Students also viewed these Sciences questions
-
The [Ni (H2O)6]2+ ion has an absorption maximum at about 725 nm, whereas the [Ni (H2O)6]2+ ion absorbs at about 570nm. Predict the color of a solution of each ion. (b) The [Ni(en)3]2+ ion occurs at...
-
in the Maller-Lyer illusion it is believed that the fin angles act as depth cues. That is from angles 105" to 165" our brains interpret it as an edge coming towards us and angles 15 to 75" appear as...
-
What products would you expect from the reaction of 1 mol of 1,3-butadiene and each of the following reagents? (If no reaction would occur, you should indicate that as well.) (a) 1 mol of Cl2 (b) 2...
-
Given that a quantity Q(t) is described by the exponential growth function Q(t) = 400e0.01t where t is measured in minutes, answer the following questions. (a) What is the growth constant k? k = (b)...
-
Consider Figure 9-10, which plots the gross domestic product (GDP) growth, in percent, against the ratio of investment/GDP, in percent, for several countries for 1974 to 1985.28 The various countries...
-
What are your thoughts on having an outsider CEO who has no experience in movie industry to lead the change at Marvel?
-
Identify the goods. Items are identified with the appropriate stock-keeping unit (SKU) number (part number) and the quantity received is recorded. LO.1
-
Cran Health Products is a cranberry cooperative that operates two divisions, a harvesting division and a processing division. Currently, all of harvestings output is converted into cranberry juice by...
-
please provide answer of this question before 11 pm today The following information as to earnings and deductions for the weekly pay period ended March 9 was taken from a com records: Required: 1....
-
The Bonavista Patient Transfer Company is considering the purchase of a new ambulance. The decision will rest partly on the anticipated distance to be driven next year. The kilo meters driven during...
-
The colors in the copper-containing minerals malachite (green) and azurite (blue) come from a single d-d transition in each compound. (a) What is the electron configuration of the copper ion in these...
-
Give the number of (valence) d electrons associated with the central metal ion in each of the following complexes: (a) K 3 [Fe(CN) 6 ] (b) [Mn(H 2 O) 6 ](NO 3 ) 2 (c) Na[Ag(CN) 2 ] (d) [Cr(NH 3 ) 4...
-
Mike's Veneer Shop owns a vacuum press that requires annual maintenance. Mike has a contract to cover the maintenance expenses for the next 5 years. The contract calls for an annual payment of \(\$...
-
Leslie Sporting Goods is a locally owned store that specializes in printing team jerseys. The majority of its business comes from orders for various local teams and organizations. While Leslie's...
-
Euclid acquires a 7-year class asset on May 9, 2022, for $153,000 (the only asset acquired during the year). Euclid does not elect immediate expensing under 179. He does not claim any available...
-
Williams & Sons last year reported sales of $10 million, cost of goods sold (COGS) of $8 million, and an inventory turnover ratio of 2. The company is now adopting a new inventory system. If the new...
-
A ceramic manufacturer promised to deliver 25 crates of vases to a Japanese importer under a "CFR" INTERCOM agreement. During transit, however, a large number of vases were broken. The buyer wants to...
-
A company receives $364, of which $23 is for sales tax. The journal entry to record the sale would include a ?
-
In 2010, RAD Partnership was organized by three equal partners: two individuals (Rachael and Adam) and Depesh Corporation. On January 10, 2011, RAD Partnership purchases 18,000 shares of qualified...
-
(a) Explain why the concentration of dissolved oxygen in freshwater is an important indicator of the quality of the water. (b) How is the solubility of oxygen in water affected by increasing...
-
Although polyethylene can twist and turn in random ways, the most stable form is a linear one with the carbon backbone oriented as shown in the following figure: The solid wedges in the figure...
-
(a) In polyvinyl chloride shown in Table 12.5, which bonds have the lowest average bond enthalpy? (b) When subjected to high pressure and heated polyvinyl chloride converts to diamond. During this...
-
Silicon has the diamond structure (Figure 12.30(a)) with unit cell edge length of 5.43 and eight atoms per unit cell. (a) How many silicon atoms are there in of material? (b) Suppose you dope that...
-
thumbs up if correct A stock paying no dividends is priced at $154. Over the next 3-months you expect the stock torpeither be up 10% or down 10%. The risk-free rate is 1% per annum compounded...
-
Question 17 2 pts Activities between affiliated entities, such as a company and its management, must be disclosed in the financial statements of a corporation as O significant relationships O segment...
-
Marchetti Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,000 cases of wine at a price of 200 euros per case. The total purchase price is 200,000...

Study smarter with the SolutionInn App


