Use the solubility-product constant for Cr(OH) 3 (K sp = 6.7 10 -31 ) and the
Question:
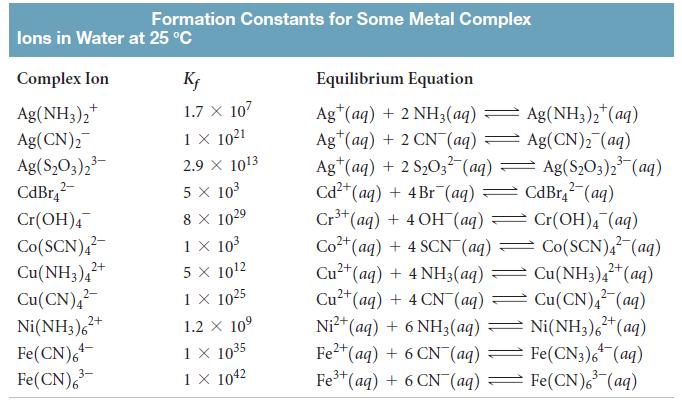
Use the solubility-product constant for Cr(OH)3 (Ksp = 6.7 × 10-31) and the formation constant for Cr(OH)4 from Table 17.1 to determine the concentration of Cr(OH)4 in a solution that is buffered at pH = 10.0 and is in equilibrium with solid Cr(OH)3.
Transcribed Image Text:
Formation Constants for Some Metal Complex lons in Water at 25 °C Complex Ion K; Equilibrium Equation Ag(NH3),* Ag(CN)2 Ag(S,O3),3- CdBr,- Ag" (aq) + 2 NH3(aq) = Ag(NH3)2*(aq) Ag*(aq) + 2 CN (aq) Ag(CN)2 (aq) Ag*(ag) + 2 S203 (aq) = Ag(S,03)2 (aq) Cd2+(aq) + 4Br (aq) Cr*(aq) + 4OH (aq) Cr(OH), (aq) Co2+(aq) + 4 SCN (aq) Cu2+(aq) + 4 NH3(aq) Cu2+(aq) + 4 CN (aq) = 2+(aq)+6 NH3(aq) 1.7 X 107 Ag(NH3),*(aq) 1 × 1021 2.9 x 1013 Ag(S,O3)2 (aq) 5 x 103 CdBr, (aq) Cr(OH)4 8 X 1029 Co(SCN),- Cu(NH3),+ Cu(CN), Ni(NH3),+ Fe(CN),- Fe(CN), 1 x 103 5 x 1012 1X 1025 Co(SCN), (aq) Cu(NH3),*(aq) Cu(CN), (aq) 2+ 2- Ni²* (aq) + 6 NH3(aq) = Ni(NH3),*(aq) Fe"(aq) + 6 CN (aq) = Fe(CN3), (aq) Fe (aq) + 6 CN (aq) 1.2 X 10° 1 X 1035 1 X 1042 4- 3- = Fe(CN), (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
The formation of complex ion CrOH4 involves two reaction steps CrOH3s Cr 3 aq ...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 978-0321696724
12th edition
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
Question Posted:
Students also viewed these Sciences questions
-
Calculate the solubility-product constant for each of the following substances, given that the molar concentrations of their saturated solutions are as indicated: (a) RaSO4 (6.6 x 10-6 M). (b)...
-
The solubility-product constant for Ce(IO3)3 is 3.2 x 10-10. What is the Ce3+ concentration in a solution prepared by mixing 50.00 mL of 0.0450 M Ce3+ with 50.00 mL of? (a) Water? (b) 0.0450 M IO3-?...
-
The solubility-product constant for barium permanganate, Ba(MnO 4 ) 2 , is 2.5 10 -10 . Assume that solid Ba(MnO 4 ) 2 is in equilibrium with a solution of KMnO 4 .What concentration of KMnO 4 is...
-
Linking every transport stakeholder together and ensuring seamless travel across Europe is a dream. With this objective, Amadeus, a leading global travel technology player, initiated a novel idea of...
-
What is wrong with this statement of purpose? PURPOSE: Determine if it takes too long to get cash from the automated teller machine during the lunch hour. Give an improved statement of purpose.
-
How does an MLP type neural network learn?
-
What do research findings tell us about motives and incentives in public organizations concerning such matters as money, security, and other motives? LO.1
-
What are noncash investing and financing activities? Provide an example. How are such transactions shown on the statement of cash flows?
-
Study the scenario below and answer the questions that follow: Coff Lounge The owner of Coff Lounge, a small business, is considering an expansion at either its Cape T Time left 2 : 5 6 : 1 5 Hide...
-
Nolas Nuts, a store that specializes in selling nuts, has available 90 pounds (lb) of cashews and 120 lb of peanuts.These are to be mixed in 12-ounce (oz) packages as follows: a lower-priced package...
-
Tooth enamel is composed of hydroxyapatite, whose simplest formula is Ca 5 (PO 4 ) 3 OH, and whose corresponding Ksp = 6.8 10 -27 .As discussed in the Chemistry and Life box on page 730, fluoride in...
-
Calculate the solubility of Mg(OH) 2 in 0.50 M NH 4 Cl.
-
Lewin Skis, Inc., today expects to earn $8.50 per share for each of the future operating periods (beginning at Time 1), today if the firm makes no new investments and returns the earnings as...
-
If the dose rate from a sample of Ga-67 is 0.052 mSv per hour at a distance of 1.1 m, then what would be dose rate at 3.5 m ?
-
A 1.6x10^9 p/s point source of Po210-Be source of 4.5 MeV is stored behind a X cm of paraffin, the dose equivalent rate is not to exceed 0.10 mSvh-1h at a distance of 1m. What is the X cm needed to...
-
X 10 Let A = -9 y 7 4 Z 210 If the kernel of A contains the vector what are x, y, and z? -2
-
8-22. E.O.Q., Carrying cost = Storing cost + Interest. Following data are available with respect to a certain material. Annual requirement.......... Cost to place an order.. Annual interest rate. _...
-
A new company started production. Job 1 was completed, and Job 2 remains in production. Here is the information from the job cost sheets from their first and only jobs so far: Job 1 Hours Total Cost...
-
Convert from degrees to radians. 900
-
Baxter, Inc., owns 90 percent of Wisconsin, Inc., and 20 percent of Cleveland Company. Wisconsin, in turn, holds 60 percent of Clevelands outstanding stock. No excess amortization resulted from these...
-
A 50-lossless line of length l = 0 375connects a 300-MHz generator with Vg = 300 V and Zg = 50 to a load ZL. Determine the time-domain current through the load for: (a) ZL =...
-
A generator with Vg = 300 V and Zg = 50 is connected to a load ZL = 75 through a 50-lossless line of length l = 0 15. (a) Compute Zin, the input impedance of the line at the...
-
If the two-antenna configuration shown in Fig. 2-41 (P2.32) is connected to a generator with Vg =250 V and Zg = 50 , how much average power is delivered to each antenna?
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


