Which arrangement of atoms in a lattice represents close-packing? (i)
Question:
Which arrangement of atoms in a lattice represents close-packing?
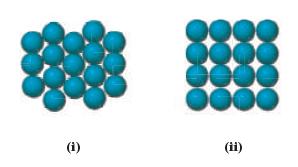
Transcribed Image Text:
(i)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
ii In a closepacked lattice the atoms are arranged in such a way as to maximize the packing effi...View the full answer

Answered By

Isabel Seraspi
I have experience teaching math, science, and English to students of all ages. I have also worked as a tutor in a college setting, helping students with their homework and preparing them for exams.
I believe that tutoring is a great way to help students learn. It allows students to get one-on-one help with their studies, and it gives them the chance to ask questions and get immediate feedback. Tutoring can also be tailored to the individual needs of the student, which is why I believe it is so effective.
I have seen firsthand how tutoring can help students improve their grades and confidence. I have also seen how it can help students who are struggling with a particular subject. I believe that tutoring is a great way to help students learn and succeed in school.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus
Question Posted:
Students also viewed these Sciences questions
-
How does the arrangement of atoms in a crystalline substance differ from the arrangement in a noncrystalline substance?
-
Atoms are found to move from one lattice position to another at the rate of 5 105 jumps/s at 400 C when the activation energy for their movement is 30,000 cal/mol. Calculate the jump rate at 750 C?
-
How does the number of atoms in a 26.5-gram gold ring compare to the number in a silver ring of the same mass?
-
Which one the below does not define "Work role boundaries" of a care worker limits that allow a patient and staff to connect safely in a therapeutic relationship based on patients' needs rules of...
-
Mrs. F, age 57, participates in the group-term life insurance plan sponsored by her corporate employer. According to Treasury tables, the cost of $1,000 of life insurance for a 57-year-old person is...
-
Shelbys Photographs has fixed operating costs of $16,500 and variable operating costs of $8 per photograph pack. The photograph packs sell for $20 each. How many photograph packs must be sold for the...
-
Dyson successfully sells its fans and heaters for $150 to $400, whereas most conventional fans sell for around $25. Explain the value it creates and how this affects the price it can charge.
-
Aragon makes handheld calculators in two models: basic and professional. Aragon estimated $851,500 of manufacturing overhead and 655,000 machine hours for the year. The basic model actually consumed...
-
2 On December 31, 2018. Alan and Company prepared an income statement and balance sheet but failed to take into account four adjusting journal entries. The income statement, prepared on this...
-
Show how the Adler algorithm (Figure 10.19) attaches weights to the data items when calculating the checksum. Figure 10.19 Start Notes L: Left 16-bit checksum R: Right 16-bit checksum D;: Next 16-bit...
-
The unit cell of a compound containing potassium, aluminum, and fluorine is shown here. (a) What type of lattice does this crystal possess (all three lattice vectors are mutually perpendicular)? (b)...
-
Indicate whether each statement is true or false: (a) The liquid crystal state is another phase of matter, just like solid, liquid, and gas. (b) Liquid crystalline molecules are generally spherical...
-
DLite Dry Cleaners is owned and operated by Joel Palk. A building and equipment are currently being rented, pending expansion to new facilities. The actual work of dry cleaning is done by another...
-
16. List I describes four systems, each with two particles A and B in relative motion as shown in figures. List II gives possible magnitude of their relative velocities (in m s) at time t = 3 S....
-
17. List I describes thermodynamic processes in four different systems. List II gives the magnitudes (either exactly or as a close approximation) of possible changes in the internal energy of the...
-
1. 2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K and 1 atm into HgO(s). During the reaction, temperature increases from 298.0 K to 312.8 K. If heat...
-
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the resulting solution is [Given: pk, and pKa2 of H2CO3 are 6.37 and 10.32,...
-
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius method, the amount of AgBr formed (in g) is [Given: Atomic mass...
-
Horst and Jurgen Romani grew up in Europe. As an adult, Horst immigrated to Vancouver. Jurgen remained behind and, while in France, developed an idea for a perfume company. He then began to purchase...
-
Smiths Family Fashions implemented a balanced scorecard performance measurement system several years ago. Smiths is a locally owned clothing retailer with fashions for men, women, teens, and...
-
(a) Calculate the standard enthalpy of formation of gaseous diborane (B2H6) using the following thermochemical information: (b) Pentaborane (B5H9) is another boron hydride. What experiment or...
-
From the following data for three prospective fuels, calculate which could provide the most energy per unit volume: Density at 20 C Molar Enthalpy of Combustion Fuel (g/cm (kJ/mol) Nitroethane, C2H...
-
The hydrocarbons acetylene (C2H2) and benzene (C6H6) have the same empirical formula. Benzene is an "aromatic" hydrocarbon, one that is unusually stable because of its structure. (a) By using the...
-
Pedro lives in Puerto Rico and had a net taxable income of $35,000 for the year 20X1. Your gross income totals $60,000. What is Pedro's regular income tax for 20X1? a.$4,620 b.$4,900 c.$2,318 d.$2,520
-
The change in cash is equal to the change in liabilities less the change in equity plus the change in noncash assets. O True False
-
Tom holds a 5-yr 10%-coupon bond, while his friend Jackson holds a 6- year 8%-coupon bond. They are both concerned about interest rate risk but they do not fully understand how it affects them. Can...

Study smarter with the SolutionInn App


