A 0.500 kg aluminum pan on a stove is used to heat 0.250 liters of water from
Question:
A 0.500 kg aluminum pan on a stove is used to heat 0.250 liters of water from 20.0°C to 80.0°C.
(a) How much heat is required? What percentage of the heat is used to raise the temperature of
(b) The pan and
(c) The water?
Strategy
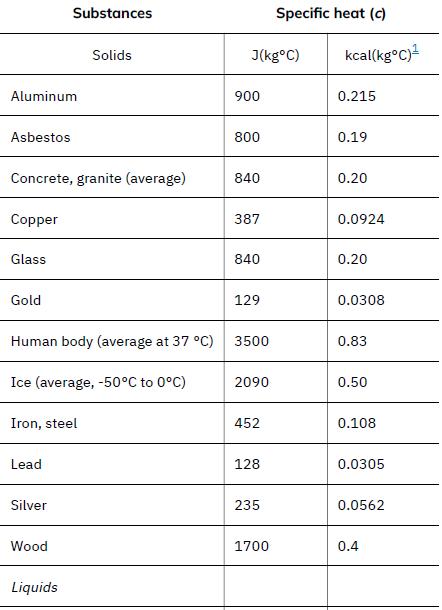
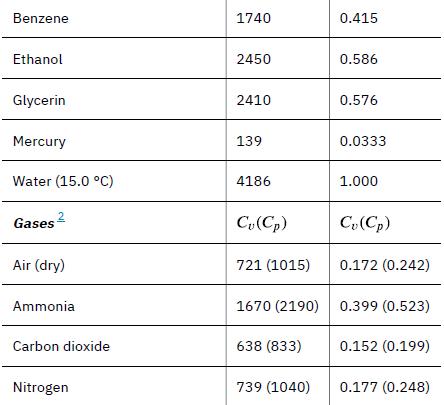
The pan and the water are always at the same temperature. When you put the pan on the stove, the temperature of the water and the pan is increased by the same amount. We use the equation for the heat transfer for the given temperature change and mass of water and aluminum. The specific heat values for water and aluminum are given in Table 14.1.
Data given in Table 14.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





