Can carbon dioxide be liquefied at room temperature (20C)? If so, how? If not, why not? (See
Question:
Can carbon dioxide be liquefied at room temperature (20°C)? If so, how? If not, why not? (See Figure 13.33.)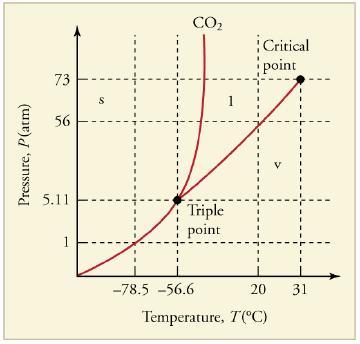
Transcribed Image Text:
Pressure, P(atm) 73 56 5.11 S CO₂ Triple point -78.5 -56.6 1 Critical point 20 31 Temperature, T(°C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
No carbon dioxide cannot be liquefied at room temperature 20C The critical point of carbon di...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-7. Ivan sold the following securities during the year and received a Form 1099-B that...
-
Review the following exhibits for Kitchenmaid: (Click the icon to view the functional cost breakdown.) (Click the icon to view the customer feature ratings.) m link the innn in this site function...
-
The investment is $1,000,000, which is depreciated straight line for 10 years down to a zero salvage value . For its 10-year life, the investment will generate annual sales of $800,000 and annual...
-
Bob Sample and other student investors opened Campus Laundromat Inc. on September 1, 2014. During the first month of operations, the following transactions occurred. The chart of accounts for the...
-
Under what conditions does imagery improve memory? Describe techniques that use imagery as a tool to improve memory. What is the basic principle that underlies these techniques? lo1
-
A social psychologist wishes to test the assertion that our attitude toward other people tends to reflect our perception of their attitude toward us. A randomly selected member of each of 12 couples...
-
If you need a better picture of something or numbers let me know and I can upload it Also if you zoom into your screen the picture should be clear ACCT 2023 PROJECT 1 Amazing Company began operations...
-
Computer Village sells computer equipment and home office furniture. Currently the furniture product line takes up approximately 50 percent of the company's retail floor space. The president of...
-
(a) If a 500-mL glass beaker is filled to the brim with ethyl alcohol at a temperature of 5.00C, how much will overflow when its temperature reaches 22.0C? (b) How much less water would overflow...
-
What is the vapor pressure of solid carbon dioxide (dry ice) at -78.5C?
-
The company intends to issue 20-year bonds with a face value of $1,000. The bonds carry a coupon rate of 9%, and interest is paid semiannually. On the issue date, the market interest rate for bonds...
-
7. Consider the design of a burglar alarm for a house. When activated an alarm and lights will be activated to encourage the unwanted guest to leave. This alarm be activated if an unauthorized...
-
A uniform flat plate of metal is situated in the reference frame shown in the figure below.
-
Q3. (a) Sketch and name the road structure layers including the bituminous coating layers. State the material's CBR value and the degree of compaction (DOC) for each road layer required by road works...
-
2. Justify the following in terms of impulse and momentum: a. Why are padded dashboards safer in automobiles?
-
The kidneys are an essential organ to regulating blood pressure in the cardiovascular system. Renal epithelial cells feature cilia on their surface, which allows them to sense blood flow in the...
-
Hedged Forecasted Purchase Mansfield Corporation purchases merchandise from a German supplier on a regular basis. On April 1, 2013, Mansfield purchased 14,000 for delivery on June 30, 2013, in...
-
What is taxable income, and what is the formula for determining taxable income?
-
What types of activities are involved in digital marketing? Choose one and explain.
-
Discuss how the business model canvas can help an entrepreneur create a framework for generating sales. Think of a business you like and describe how it generates sales.
-
How does guerrilla marketing differ from viral marketing?
-
true- false statement (e) The objective of a family foundation is charity. (f) Limited liability offers bankruptcy protection to shareholders
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
You just won a stock picking contest that will pay you $24,000 a year for 26 years, and you get the first payment today. What is the prize worth to you today if your annual opportunity cost rate is...

Study smarter with the SolutionInn App


