Find the energy emitted in the decay of 239 Pu. Strategy Nuclear reaction energy, such as
Question:
Find the energy emitted in the α decay of 239Pu.
Strategy
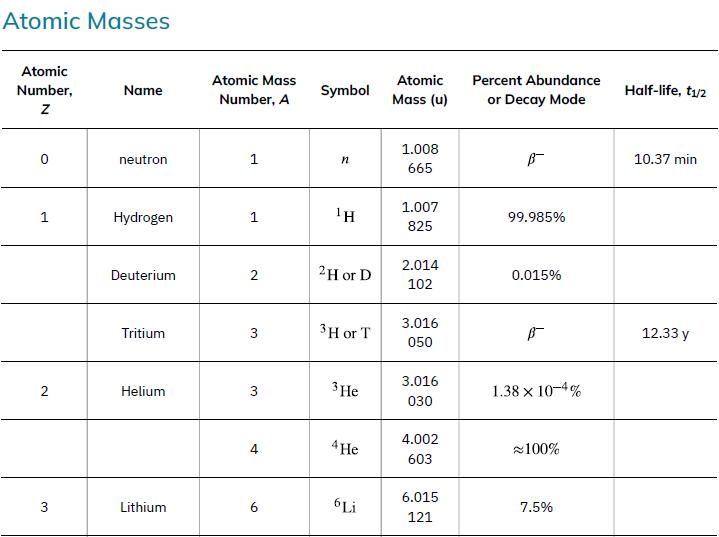
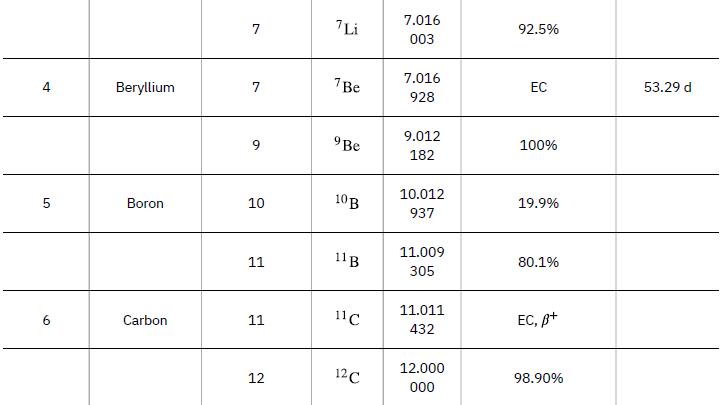
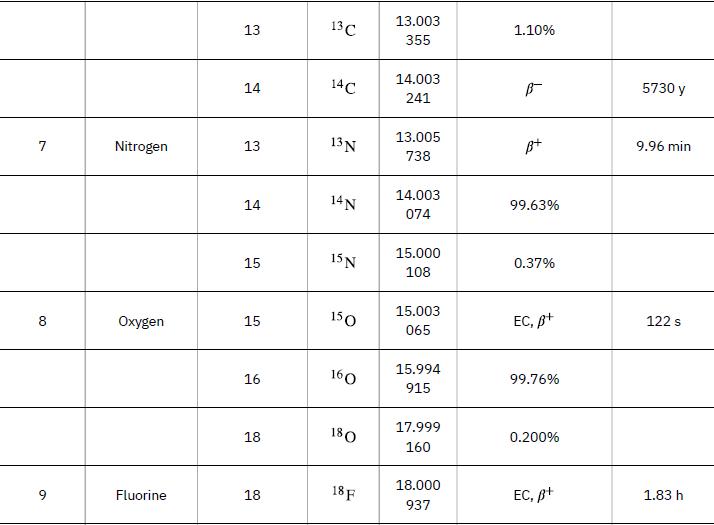
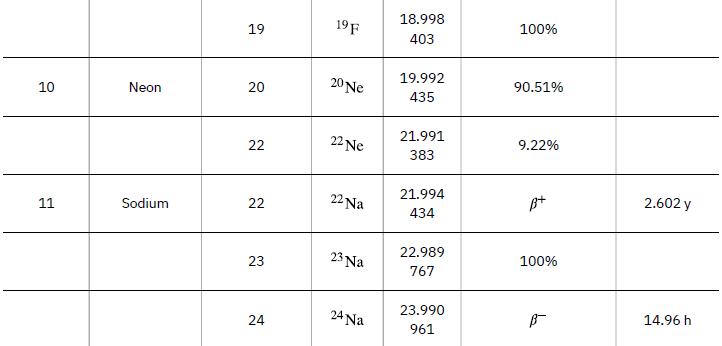
Nuclear reaction energy, such as released in a decay, can be found using the equation E =(Δm)c2. We must first find Δm, the difference in mass between the parent nucleus and the products of the decay. This is easily done using masses given in Appendix A.
Data from Appendix A
Transcribed Image Text:
Atomic Masses Atomic Number, Z 1 2 3 Name neutron Hydrogen Deuterium Tritium Helium Lithium Atomic Mass Number, A 1 1 2 3 3 4 9 Symbol ΤΗ 2H or D 3 H or T 3 He 4 He 6 Li Atomic Mass (u) 1.008 665 1.007 825 2.014 102 3.016 050 3.016 030 4.002 603 6.015 121 Percent Abundance or Decay Mode B- 99.985% 0.015% B- 1.38 x 10-4% ≈100% 7.5% Half-life, t₁/2 10.37 min 12.33 y
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
The decay equation was given earlier for 239 Pu it is Thus the pertinent masses are those of 239 Pu ...View the full answer

Answered By

DEEPTI A.
Hi
I am a Chartered Accountant (Indian CPA) and an MBA Finance with 18+ years of work experience. I am the owner of a small accounting firm. I provide bookkeeping and accounting support to my clients located in different countries. Together with it, I also teach students and help in solving and completing their accounting-related questions and projects.
My core area of expertise lies in teaching students
1) Accountancy
2) Finance
I teach theoretical subjects too but the above 2 are my favorites. My motto is to have an interactive class and design ways and means to make the concept interesting for my students.
Before starting off with teaching I have worked with different MNC's like GECIS, HSBC and IBM and been a part of their Finance, Accounting, and Financial Analytic teams.
So do provide your questions/queries and I'll be more than happy to answer those and hope to help you in whatever manner I can.
All the best in your endeavors!
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
Find the energy emitted in the - decay of 60 Co. Strategy and Concept As in the preceding example, we must first find m, the difference in mass between the parent nucleus and the products of the...
-
One of the waste products of a nuclear reactor is plutonium-239 ( 239 Pu). This nucleus is radioactive and decays by splitting into a helium-4 nucleus and a uranium-235 nucleus ( 4 He + 235 U), the...
-
50 successes in 200 trials when p = 0.2. For the binomial experiments find the normal approximation for the probability of
-
What is the formula for calculating return on investment (ROI)?
-
Kenmare Architects Ltd. (KAL) was incorporated and commenced operations on January 1, 2014. Sheila Kenmare, the company's only employee, consults with various clients and uses expensive equipment to...
-
In problem 2.2, how much will be produced in each of the 3 months? LO.1
-
The following balances appear on the books of Sarah Simon Enterprises: Retained Earnings, $ 26,100; Dividends, $ 8,500; Service Revenue, $ 23,700; Salaries Expense, $ 6,100; Rent Expense, $ 4,000;...
-
Indicate whether the general ledger accounts will be debited or credited when recording the following entries: Issue stationery from the store for use to an employee Inventory: DEBIT Inventory: CREDIT
-
(a) For carbon, calculate the energy when an electron falls from n = 3 to n = 2, then from n = 2 to n = 1. Then add these for the total energy released in the process. (b) Calculate the energy...
-
The solar corona is so hot that most atoms in it are ionized. Consider a hydrogen-like atom in the corona that has only a single electron. Construct a problem in which you calculate selected spectral...
-
In the following summary of data for a payroll period, some amounts have been intentionally omitted: Earnings: 1. At regular rate...............................? 2. At overtime...
-
Recognition is a very important element of volunteer management. Do you know someone who has done amazing volunteer work for a good cause? Wouldn't it be nice to thank them with an award! Take a look...
-
What do Financial Planners do? Would you consider hiring a Financial Planner? How important are ethics when working with a financial planning professional? Explain the concept of return on...
-
Explain the specific perceptual errors you made of EACH of your teammates during the class exercise
-
Identify the company that makes the product a. Are they a large company or a small company? b. Are they a chain or a major corporation? c. Have they been around for decades or are they a new company?...
-
The Red Inn at Pismo is a 150-room hotel that caters mainly to business clients during the week and to tourists during the weekends and in the summer. Below is a table summarizing the average daily...
-
The angle between the two equal sides of an isosceles triangle measures 0.53 0.005 radian. The two equal sides are exactly 151 centimeters long. Calculate the length of the third side with an...
-
A consumer magazine is evaluating five brands of trash compactors for their effectiveness in reducing the volume of typical household products that are discarded. In the experiment, each block...
-
A tennis player hits a ball 2.0 m above the ground. The ball leaves his racquet with a speed of 20.0 m/s at an angle 5.0 above the horizontal. The horizontal distance to the net is 7.0 m, and the net...
-
While a person is walking, his arms swing through approximately a 45 angle in s. As a reasonable approximation, we can assume that the arm moves with constant speed during each swing. A typical arm...
-
Human Biomechanics. The fastest pitched baseball was measured at Typically, a baseball has a mass of 145 g. If the pitcher exerted his force (assumed to be horizontal and constant) over a distance of...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


