Question: Neutrons from a source (perhaps the one discussed in the preceding problem) bombard natural molybdenum, which is 24 percent 98 Mo. What is the energy
Neutrons from a source (perhaps the one discussed in the preceding problem) bombard natural molybdenum, which is 24 percent 98Mo. What is the energy output of the reaction ![]() ? The mass of 98Mo is given in Appendix A: Atomic Masses, and that of 99Mo is 98.907711 u.
? The mass of 98Mo is given in Appendix A: Atomic Masses, and that of 99Mo is 98.907711 u.
Data from Appendix A
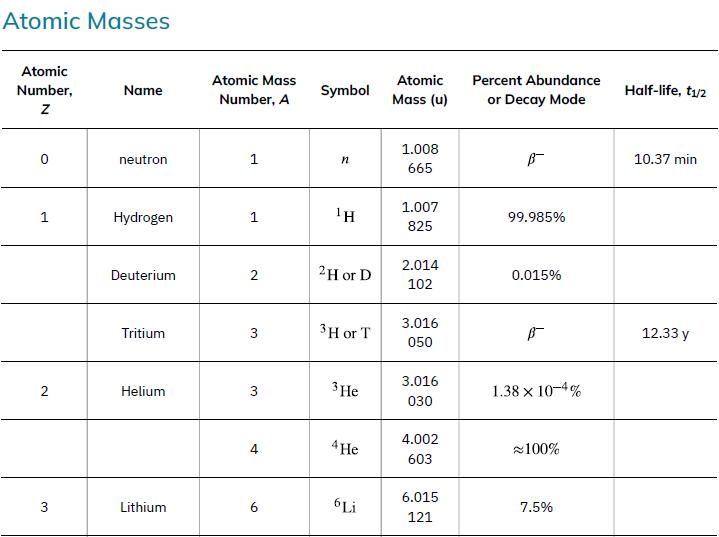
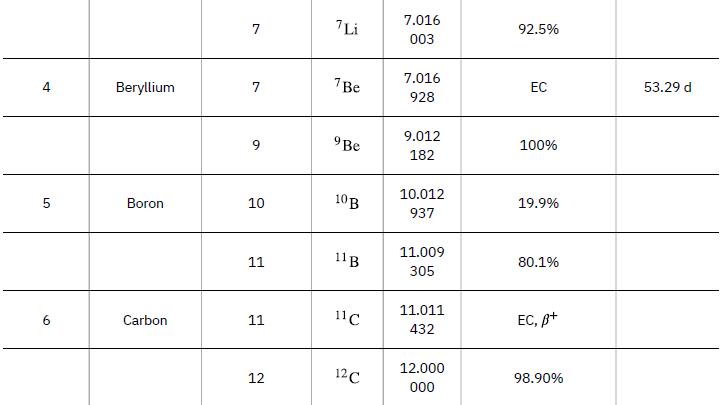
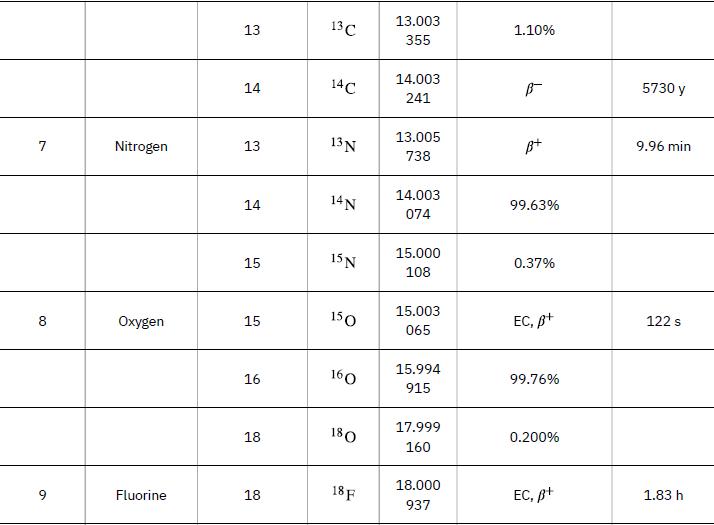
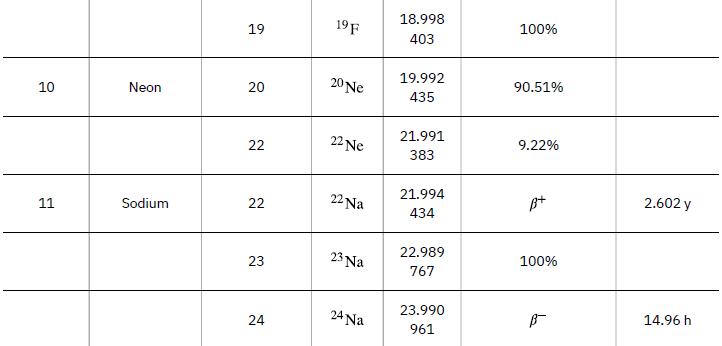
98 Mo + n 99 Mo + y Y
Step by Step Solution
3.30 Rating (162 Votes )
There are 3 Steps involved in it
The nuclear reaction 98Mon My 98 Mo 97905406 u ... View full answer

Get step-by-step solutions from verified subject matter experts


