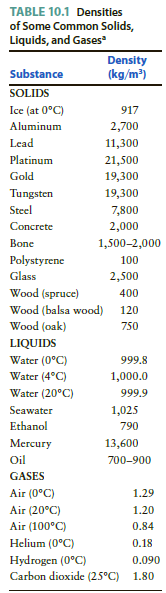
Add a new column to Table 10.1, listing the specific gravity of each substance. TABLE 10.1 Densities
Question:
Add a new column to Table 10.1, listing the specific gravity of each substance.
Transcribed Image Text:
TABLE 10.1 Densities of Some Common Solids, Liquids, and Gases Density (kg/m) Substance SOLIDS Ice (at 0°C) 917 Aluminum 2,700 Lead 11,300 Platinum 21,500 Gold 19,300 Tungsten 19,300 Steel 7,800 Concrete 2,000 Bone 1,500-2,000 Polystyrene 100 Glass 2,500 Wood (spruce) 400 Wood (balsa wood) 120 Wood (oak) 750 LIQUIDS Water (0°C) 999.8 Water (4°C) 1,000.0 Water (20°C) 999.9 Seawater 1,025 Ethanol 790 Mercury 13,600 Oil 700-900 GASES Air (0°C) 1.29 Air (20°C) 1.20 Air (100°C) 0.84 Helium (0°C) 0.18 Hydrogen (0°C) Carbon dioxide (25°C) 1.80 0.090
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
Apply the concepts of density and specific gravity No sketch needed Specific gravity is the ratio of ...View the full answer

Answered By

Mary Njunu
I posses Vast, diversified knowledge and excellent grammar as a result of working in ACADEMIC WRITING for more than 5 years. I deliver work in various disciplines with assurance of quality work. I purpose at meeting the clients’ expectations precisely. Let’s work together for the best and phenomenal grades.
4.90+
929+ Reviews
2557+ Question Solved
Related Book For 

College Physics Reasoning and Relationships
ISBN: 978-0840058195
2nd edition
Authors: Nicholas Giordano
Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
Add a column to Table 10.1 describing the appearance of these plastics, including available colors and opaqueness?
-
The specific gravity of benzene is 0.876. Calculate its specific weight and its density in SI units.
-
The specific gravity of benzene is 0.876. Calculate its specific weight and its density in U.S. Customary System units.
-
Consider two mutually exclusive investment projects: A 1 and A2. Each project has the same service life, and the present worth of each component value (B, I, and C) is computed at 10% as follows:...
-
Suppose you know that a company's stock currently sells for $58 per share and the required return on the stock is 12 percent. You also know that the total return on the stock is evenly divided...
-
What is the difference between an exclusive arc and an inclusive arc?
-
P2-9 Prepare allocation schedules under different stock price assumptions (bargain purchase) Pop Corporation exchanged 40,000 previously unissued no par common shares for a 40 percent interest in Son...
-
Kelly Services (Kelly) places employees at clients businesses on a temporary basis. It segments its services into (1) Commercial, (2) Professional and technical(3) International. Kelly recognizes...
-
0 Required information The Foundational 15 (Algo) (LO8-2, LO8-3, L08-4, LO8-5, LOB-7, LO8-9, LO8-10) [The following information applies to the questions displayed below.) Morganton Company makes one...
-
Steve and Jane Mitchell, both 35 years old, met and married. They each embarked on promising, demanding and successful executive careers in money management after grad school. Their goal is to retire...
-
Explain how the specific gravity of a substance is connected with the buoyant force when an object is immersed in water.
-
The region near the South Pole in Antarctica has an altitude of about 3000 m, making the air pressure lower than at sea level. Explain why it is more difficult for an airplane to take off from the...
-
The mass 82 isotope of bromine (82Br) is radioactive and is used as a tracer to identify the origin and destination of individual atoms in chemical reactions and biological transformations. A sample...
-
In 2024, the Westgate Construction Company entered into a contract to construct a road for Santa Clara County for $10,000,000. The road was completed in 2026. Information related to the contract is...
-
Briefly describe the case you have chosen. Categorize the social worker's experience as vicarious trauma, compassion fatigue, or burnout. Provide justification. Identify the social worker's score on...
-
Given f(x) below, find f'(x). f(x) = = m 5z In (2) et dt
-
Olsen & Alain, CPAs (O&A) performed the audit of Rocky Point Brewery (RPB), a public company in 20X1 and 20X2. In 20X2, O&A also performed tax services for the company. Which statement best describes...
-
Exercise 9-4 (Algo) Prepare a Flexible Budget Performance Report [LO9-4] Vulcan Flyovers offers scenic overflights of Mount Saint Helens, the volcano in Washington State that explosively erupted in...
-
What is a market? What are the requirements for a market?
-
Show that the block upper triangular matrix A in Example 5 is invertible if and only if both A 11 and A 22 are invertible. Data from in Example 5 EXAMPLE 5 A matrix of the form A = [ A11 A12 0 A22 is...
-
By substituting in the Schrdinger equation for the harmonic oscillator, shows that the ground-state vibrational wave function is an eigenfunction of the total energy operator. Determine the energy...
-
Evaluate the average linear momentum of the quantum harmonic oscillator, px , for the ground state (n = 0) and first two excited states (n = 1and n = 2).
-
By substituting in the Schrdinger equation for rotation in three dimensions, show that the rotational wave function (5/16) 1/2 (3cos 2 1) is an eigenfunction of the total energy operator. Determine...
-
A contractor constructed a house for resale, which was sold immediately. For tax purposes, this is an example of A) capital income. B) business income. C) other income. D) property income.
-
You invest $100 in a risky asset with an expected rate of return of 0.12 and a standard deviation of 0.15 and a T-bill with a rate of return of 0.05. What percentages of your money must be invested...
-
Nanometrics, Inc., has a beta of 3.43. If the market return is expected to be 13.50 percent and the risk-free rate is 7.00 percent, what is Nanometrics required return? (Round your answer to 2...

Study smarter with the SolutionInn App


