Find the center of mass of the two particles in Figure P7.41. Figure P7.41 ? 7.0 kg
Question:
Find the center of mass of the two particles in Figure P7.41.
Figure P7.41
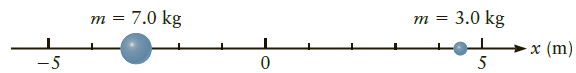 ?
?
Transcribed Image Text:
7.0 kg 3.0 kg т т х (m) -5 5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 93% (16 reviews)
The center of mass of a system of particles can be found by multiplying ...View the full answer

Answered By

Navashree Ghosh
I believe in quality work and customer satisfaction. So, I can assure you that you will get quality work from me when you hire me. Let's work together and build a long-term association.
4.90+
82+ Reviews
116+ Question Solved
Related Book For 

College Physics Reasoning and Relationships
ISBN: 978-0840058195
2nd edition
Authors: Nicholas Giordano
Question Posted:
Students also viewed these Sciences questions
-
Find the center of mass of the four particles in Figure P7.42. Their masses are m 1 = 12 kg, m 2? = 18 kg, m 3 = 7.9 kg, and m 4 = 23 kg. Figure P7.42 ? y (m). -10 -5. (m) 10- -5. m2
-
Find the center of mass of a uniformly solid cone of base diameter 2a and height h and a solid hemisphere of radius a where the two bases are touching.
-
Find the rotational inertia of the system of point particles shown in the figure assuming the system rotates about the (a) x -axis, (b) y -axis, (c) z -axis. The z -axis is perpendicular to the xy...
-
1. Find the probability of obtaining between 40 and 60 heads when tossing a coin 100 times. 2. Find the probability of obtaining 6s between 20 and 40 times when rolling a die 200 times.
-
Some economists think the government should help fund local sports stadiums while other economists think the government should leave the funding up to the private sector. a. What kind of claims are...
-
In the presence of a platinum catalyst, ammonia, NH3, burns in oxygen, O2, to give nitric oxide, NO, and water vapor. How many volumes of nitric oxide are obtained from one volume of ammonia,...
-
What are the three major sources of internal pressure for organizational change? In your opinion, which of these three is most difficult to handle? Why?p. 477
-
Financial statement information about four different companies is as follows. Instructions (a) Determine the missing amounts. (b) Prepare the retained earnings statement for Stills Company. Assume...
-
Describe the four stages of a product life cycle and explain what strategies you are likely to adopt to promote and sustain the demand for a product as it moves through each stage of the life cycle
-
Draw an inheritance hierarchy for students at a university similar to the hierarchy shown in Fig. 9.2. Use Student as the superclass of the hierarchy, then extend Student with classes Undergraduate...
-
Two objects are moving along the x axis with velocities of 35 m/s (object 1) and -25 m/s (object 2). (a) If the center of mass has a velocity of +10 m/s, which object has the greater mass? (b) What...
-
A railroad car containing explosive material is initially traveling south at a speed of 5.0 m/s on level ground. The total mass of the car plus explosives is 3.0 10 4 kg. An accidental spark ignites...
-
Explain why Game chose the countries it entered and why in that order. LO13
-
Beginning with Eq. (11.16), prove that Data from Eq. 11.16 Data from Eq. 11.21 where we have defined D8 = - 3 2 F = FiFi T = F + F + F Y = F8. 3 Show that this leads to Eq. (11.21) with the...
-
Consider the light bulb that is the object in Figure 33.28. If you move the bulb to the left, does the image shift left, shift right, or stay in the same place? Data from Figure 33.28 (a) The three...
-
Two models of light emitted from a light bulb are illustrated in Figure P33.5. (a) Describe the difference in the behavior of light in each model. (b) Describe an experiment that can determine which...
-
Parallel red and green laser rays are incident on a glass slab as shown in Figure P33.24. Sketch the rays as they pass through the slab and after they have entered the air to the right of the slab....
-
Consider the following five operations: constructing a luxury cruise ship, operating a casual dining restaurant, staging a professional sports match, manufacturing a patented drug, and rescuing...
-
Allison invests $15,000 in an account paying 7% per year compounded annually. (a) How many years are required for the compound amount to at least double? (b) In how many years will the amount at...
-
The packaging division of a company having considered several alternative package designs for the company's new product has finally brought down their choices to two designs of which only one has to...
-
At what temperature does the slope of the z versus P curve as P 0 have its maximum value for a van der Waals gas? What is the value of the maximum slope?
-
Calculate the density of O 2 (g) at 480. K and 280. bar using the ideal gas and the van der Waals equations of state. Use a numerical equation solver to solve the van der Waals equation for V m or...
-
Show that T = 1 + T ( In z/T) P , and that Pk = 1 P( In z/T) T.
-
Which of the following accounts will not be closed during the closing process? a. Accounts Recelvable b. Wages Expense c. Fees Earned d. Rent Expense
-
Clarkson Lumber Company After a rapid growth in its business during recent years, the Clarkson Lumber Company, in the spring of 1996, anticipated a further substantial increase in sales. Despite good...
-
How do external factors such as changing consumer preferences affect the retail industry?"

Study smarter with the SolutionInn App


