Photon P in Figure Q28.35 moves an electron from energy level (n=1) to energy level (n=3). The
Question:
Photon P in Figure Q28.35 moves an electron from energy level \(n=1\) to energy level \(n=3\). The electron jumps down to \(n=2\), emitting photon \(\mathrm{Q}\), and then jumps down to \(n=1\), emitting photon \(\mathrm{R}\). The spacing between energy levels is drawn to scale. What is the correct relationship among the wavelengths of the photons?
A. \(\lambda_{\mathrm{P}}<\lambda_{\mathrm{Q}}<\lambda_{\mathrm{R}}\)
B. \(\lambda_{\mathrm{R}}<\lambda_{\mathrm{P}}<\lambda_{\mathrm{Q}}\)
C. \(\lambda_{\mathrm{Q}}<\lambda_{\mathrm{P}}<\lambda_{\mathrm{R}}\)
D. \(\lambda_{\mathrm{R}}<\lambda_{\mathrm{Q}}<\lambda_{\mathrm{P}}\)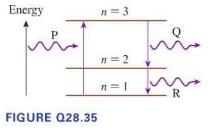
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

College Physics A Strategic Approach
ISBN: 9780321907240
3rd Edition
Authors: Randall D. Knight, Brian Jones, Stuart Field
Question Posted:





