Question: Use the data in Appendix D to calculate the chemical atomic mass of lithium, to two decimal places. Appendix D Atomic and Nuclear Data Mass
Use the data in Appendix D to calculate the chemical atomic mass of lithium, to two decimal places.
Appendix D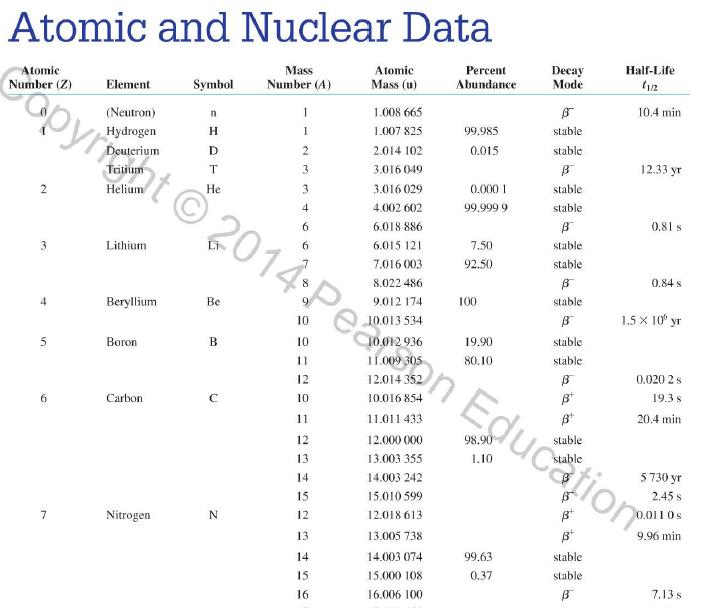
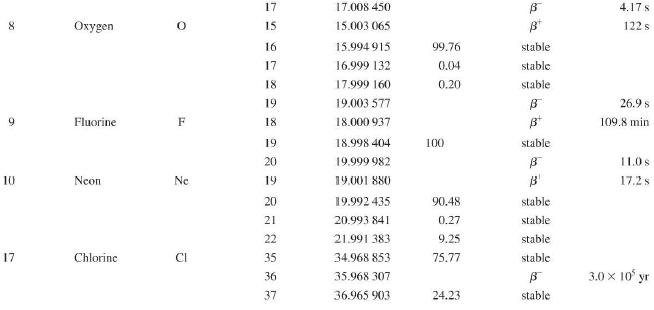
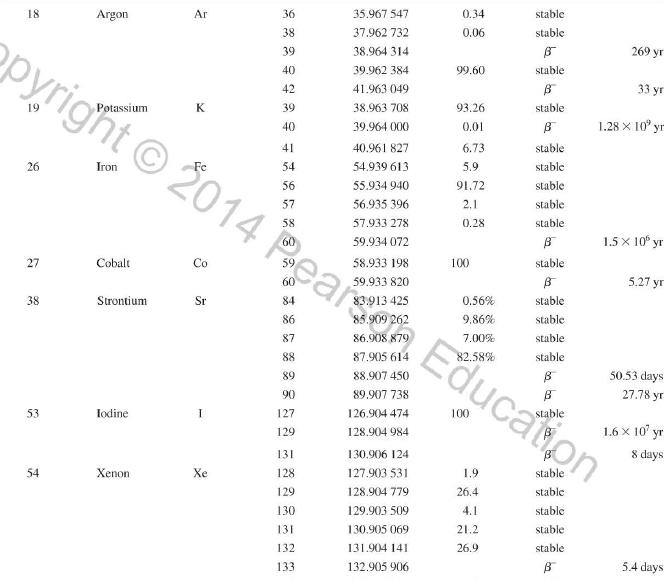
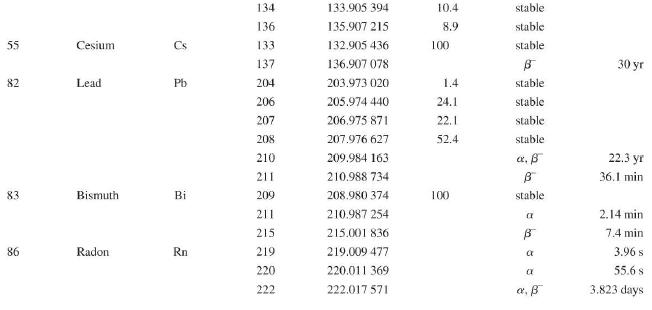
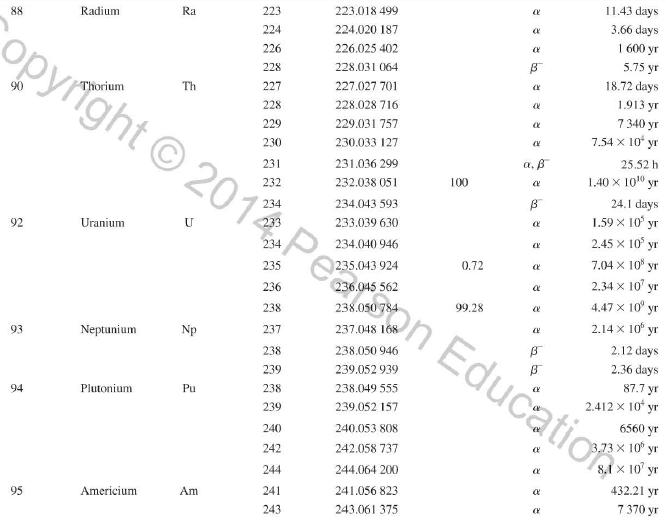
Atomic and Nuclear Data Mass Element Symbol Number (A) (Neutron) n H 1 D 2 T 3 3 4 6 3 Lithium Beryllium Be Copyright 2014 Pearson Education. Atomic Mass (u) Percent Abundance Decay Half-Life Mode (1/2 1.008 665 15 10,4 min 1.007 825 99.985 stable 2.014 102 0.015 stable 3.016 049 B 12.33 yr 3.016 029 0.0001 stable 4.002 602 99.999 9 stable 6.018 886 B 0.81 s 6.015 121 7.016 003 7.50 stable 92.50 stable 8.022 486 9.012 174 100 stable 0.84 s B 1.5 x 10" yr 5 Boron B 10 11 12 Carbon C 10 11 11.011 433 B 20.4 min 12 12.000 000 13 13.003 355 14 14.003 242 5 730 yr 15 15.010599 7 Nitrogen N 12 12.018 613 13 13.005 738 B+ 9.96 min 14 14.003 074 99.63 stable 15 15.000 108 0.37 stable 16 16.006 100 B 7.13 s 19.90 stable 80.10 stable B ba ta ba 0.020 2 s 19.3 s
Step by Step Solution
3.53 Rating (150 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


