Question: When the electron is struck by the (x)-ray photon, A. Its de Broglie wavelength increases. B. Its de Broglie wavelength decreases. C. Its de Broglie
When the electron is struck by the \(x\)-ray photon,
A. Its de Broglie wavelength increases.
B. Its de Broglie wavelength decreases.
C. Its de Broglie wavelength stays the same.
Further support for the photon model of electromagnetic waves comes from Compton scattering, in which \(\mathrm{x}\) rays scatter from electrons, changing direction and frequency in the process. Classical electromagnetic wave theory cannot explain the change in frequency of the \(\mathrm{x}\) rays on scattering, but the photon model can.
Suppose an \(\mathrm{x}\)-ray photon is moving to the right. It has a collision with a slow-moving electron, as in Figure P28.85. The photon transfers energy and momentum to the electron, which recoils at a high speed. The \(\mathrm{x}\)-ray photon loses energy, and the photon energy formula \(E=h f\) tells us that its frequency must decrease. The collision looks very much like the collision between two particles.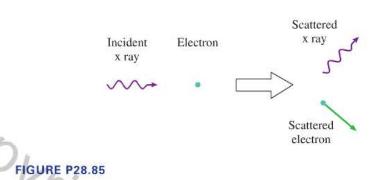
FIGURE P28.85 Incident x ray Electron Scattered x ray Scattered electron
Step by Step Solution
3.38 Rating (151 Votes )
There are 3 Steps involved in it
B Its d... View full answer

Get step-by-step solutions from verified subject matter experts


