a. The table gives the melting and boiling points for lead and oxygen. i. At 450 C
Question:
a. The table gives the melting and boiling points for lead and oxygen.
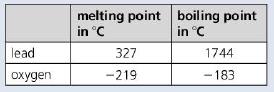
i. At 450 °C will the lead be a solid, a liquid or a gas?
ii. At -200 °C will the oxygen be a solid, liquid or a gas?
b. The graph shows how the temperature of a pure substance changes as it is heated.
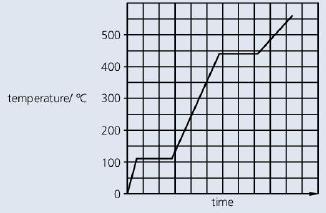
i. At what temperature does the substance boil?
ii. Sketch the graph and mark with an X any point where the substance exists as both a liquid and gas at the same time.
c. i* All substances consist of particles. What happens to the average kinetic energy of these particles as the substance changes from a liquid to a gas?
ii. Explain, in terms of particles, why energy must be given to a liquid if it is to change to a gas.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:




